对氯苯肼盐酸盐
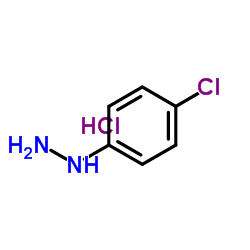
对氯苯肼盐酸盐结构式

|
常用名 | 对氯苯肼盐酸盐 | 英文名 | 4-Chlorophenylhydrazine hydrochloride |
|---|---|---|---|---|
| CAS号 | 1073-70-7 | 分子量 | 179.047 | |
| 密度 | 1.32g/cm3 | 沸点 | 265.3ºC at 760mmHg | |
| 分子式 | C6H8Cl2N2 | 熔点 | 216 °C (dec.)(lit.) | |
| MSDS | 中文版 美版 | 闪点 | 114.2ºC | |
| 符号 |

GHS07 |
信号词 | Warning |
|
Reaction of 3-acetonyl-5-cyano-1,2,4-thiadiazole with phenylhydrazine hydrochlorides: indolization and phenylpyrazolation.
Chem. Pharm. Bull. 48(1) , 160-2, (2000) Treatment of 3-acetonyl-5-cyano-1,2,4-thiadiazole (1) with 4-methyl or 4-methoxyphenylhydrazine hydrochloride provided 5-cyano-3-(2,5-dimethylindol-3-yl)-1,2,4-thiadiazole (2) or 5-cyano-3-(5-methoxy-2-methylindol-3-yl)-1,2,4-thiadiazole (3) as the sole produ... |
|
|
Reactions of prostaglandin H synthase with monosubstituted hydrazines and diazenes. Formation of iron(II)-diazene and iron(III)-sigma-alkyl or iron(III)-sigma-aryl complexes.
Eur. J. Biochem. 226(2) , 445-57, (1994) The reaction of p-chlorophenylhydrazine with prostaglandin H synthase (PGHS) Fe(III) under aerobic conditions leads to a partial destruction of the heme and to a new complex absorbing at 436 nm. This complex is also obtained by reaction of p-chlorophenyldiaze... |
|
|
Design, synthesis and biological evaluation of indane-2-arylhydrazinylmethylene-1,3-diones and indol-2-aryldiazenylmethylene-3-ones as beta-amyloid aggregation inhibitors.
Eur. J. Med. Chem. 45(4) , 1359-66, (2010) Biological screening of (hetero)aromatic compounds allowed the identification of some novel inhibitors of Abeta(1-40) aggregation, bearing indane and indole rings as common scaffolds. Molecular decoration of lead compounds led to inhibitors exhibiting a poten... |
|
|
Synthesis and in vitro binding of N,N-dialkyl-2-phenylindol-3-yl-glyoxylamides for the peripheral benzodiazepine binding sites.
Bioorg. Med. Chem. 14(11) , 3938-46, (2006) A series of N,N-dialkyl-2-phenylindol-3-ylglyoxylamides bearing the halogens iodine and bromine were synthesised and their binding affinity for the peripheral benzodiazepine binding sites (PBBS) in rat kidney mitochondrial membranes was evaluated using [(3)H]... |