频那酮
一般危化品
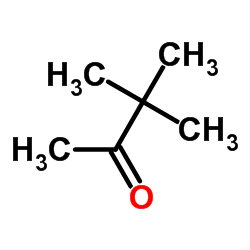
频那酮结构式
|
常用名 | 频那酮 | 英文名 | Pinacolone |
|---|---|---|---|---|
| CAS号 | 75-97-8 | 分子量 | 100.159 | |
| 密度 | 0.8±0.1 g/cm3 | 沸点 | 104.9±8.0 °C at 760 mmHg | |
| 分子式 | C6H12O | 熔点 | -52.5 °C | |
| MSDS | 中文版 美版 | 闪点 | 23.9±0.0 °C | |
| 符号 |


GHS02, GHS07 |
信号词 | Danger |
|
A novel carbonyl reductase with anti-Prelog stereospecificity from Acetobacter sp. CCTCC M209061: purification and characterization.
PLoS ONE 9(4) , e94543, (2014) A novel carbonyl reductase (AcCR) catalyzing the asymmetric reduction of ketones to enantiopure alcohols with anti-Prelog stereoselectivity was found in Acetobacter sp. CCTCC M209061 and enriched 27.5-fold with an overall yield of 0.4% by purification. The en... |
|
|
Isolation and characterization of 4-tert-butylphenol-utilizing Sphingobium fuliginis strains from Phragmites australis rhizosphere sediment.
Appl. Environ. Microbiol. 76(20) , 6733-40, (2010) We isolated three Sphingobium fuliginis strains from Phragmites australis rhizosphere sediment that were capable of utilizing 4-tert-butylphenol as a sole carbon and energy source. These strains are the first 4-tert-butylphenol-utilizing bacteria. The strain ... |
|
|
Enhancement of hepatic microsomal esterase activity following soman pretreatment in guinea pigs.
Biochem. Pharmacol. 46(11) , 2083-92, (1993) Soman (pinacolyl methylphosphonofluoridate), a highly toxic organophosphate compound, has been found to be a strong inhibitor of hepatic microsomal carboxylesterase in vitro, but an enhancer of carboxylesterase when administered in vivo. In response to this p... |
|
|
Direct conversion of arylamines to pinacol boronates: a metal-free borylation process.
Angew. Chem. Int. Ed. Engl. 49(10) , 1846-9, (2010)
|
|
|
Pinacol coupling reaction of beta-halo-alpha,beta-unsaturated aldehydes promoted by TiI4.
Org. Lett. 4(23) , 4097-9, (2002) The pinacol reaction of beta-halogenated alpha,beta-unsaturated aldehydes was promoted by titanium tetraiodide to give coupling products in good yields with high dl-selectivity. Subsequent reduction with H(2)/Pd-C gave saturated vic-diols in good yields. Heck... |
|
|
Hg(OTf)2-catalyzed vinylogous semi-pinacol rearrangement leading to 1,4-dihydroquinolines.
Org. Lett. 14(5) , 1222-5, (2012) An efficient method for the construction of dihydroquinoline derivatives possessing a quaternary carbon center is developed by an application of Hg(OTf)(2)-catalyzed vinylogous semi-pinacol-type rearrangement. The reaction was found to be specifically catalyz... |
|
|
Synthesis, spectroscopic, and in vitro photosensitizing efficacy of ketobacteriochlorins derived from ring-B and ring-D reduced chlorins via pinacol-pinacolone rearrangement.
J. Org. Chem. 76(21) , 8629-40, (2011) In this report, we present a regioselective oxidation of a series bacteriochlorins, which on reacting with either ferric chloride (FeCl(3)) or 2,3-dichloro-5,6-dicyanobenzoquinone (DDQ) yielded the corresponding ring-B or ring-D reduced chlorins. The effect o... |
|
|
Coupling of enantioselective biooxidation of DL-1,2-propanediol and bioreduction of pinacolone via regeneration cycle of coenzyme.
Appl. Microbiol. Biotechnol. 71(6) , 819-23, (2006) Enantioselective biotransformation of DL-1,2-propanediol to D-2-hydroxypropanic acid was first reported by the authors. In the biooxidation process, there were some by-product formed and thus influenced the e.e. value and output of the acid. Restricting oxyge... |
|
|
Effect of meso-substituents on the osmium tetraoxide reaction and pinacol-pinacolone rearrangement of the corresponding vic-dihydroxyporphyrins.
J. Org. Chem. 66(11) , 3930-9, (2001) To investigate the effects of electron-donating and electron-withdrawing substituents upon the reaction of porphyrins with osmium tetraoxide, and the pinacol-pinacolone rearrangement of the resulting diols, a series of meso-substituted porphyrins were prepare... |
|
|
Solvent-washable polymer templated synthesis of mesoporous materials and solid-acid nanocatalysts in one-pot.
Chem. Commun. (Camb.) (41) , 6201-3, (2009) We report a new and simple one-pot synthetic method to produce mesoporous silica and nanoporous solid acid catalyst capable of catalyzing pinacole-pinacolone rearrangement and esterification reactions, by preparing a solvent washable phosphonated triblock cop... |