碘代乙酸酐
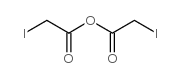
碘代乙酸酐结构式

|
常用名 | 碘代乙酸酐 | 英文名 | iodoacetic anhydride |
|---|---|---|---|---|
| CAS号 | 54907-61-8 | 分子量 | 353.88200 | |
| 密度 | 2.664g/cm3 | 沸点 | 276.9ºC at 760 mmHg | |
| 分子式 | C4H4I2O3 | 熔点 | 47-49ºC(lit.) | |
| MSDS | 中文版 美版 | 闪点 | >230 °F | |
| 符号 |


GHS05, GHS06 |
信号词 | Danger |
|
Synthesis and evaluation of nuclear targeting peptide-antisense oligodeoxynucleotide conjugates.
Bioconjug. Chem. 6(1) , 101-8, (1995) An endogenous nuclear enzyme, RNase H, is an important component in determining the efficacy of antisense oligodeoxynucleotides (ODNs). In an effort to improve the potency of antisense ODNs, conjugates with three different nuclear targeting signal peptides we... |
|
|
A novel method for the incorporation of glycoprotein-derived oligosaccharides into neoglycopeptides.
Bioconjug. Chem. 3(5) , 391-6, (1992) We describe a new method for the transfer of carbohydrate moieties to polypeptides in which complex carbohydrate, in the form of glycosyl amino acid, is removed from an available glycoprotein, derivatized, and reacted with a polypeptide via an iodoacetylated ... |
|
|
Peptide affinity chromatography media that bind N(pro) fusion proteins under chaotropic conditions.
J. Chromatogr. A. 1217(40) , 6203-13, (2010) To design a generic purification platform and to combine the advantages of fusion protein technology and matrix-assisted refolding, a peptide affinity medium was developed that binds inclusion body-derived N(pro) fusion proteins under chaotropic conditions. P... |
|
|
A general method for highly selective cross-linking of unprotected polypeptides via pH-controlled modification of N-terminal alpha-amino groups.
Bioconjug. Chem. 1(2) , 114-22, (1990) A method is described for the highly selective modification of the alpha-amino groups at the N-termini of unprotected peptides to form stable, modified peptide intermediates which can be covalently coupled to other molecules or to a solid support. Acylation w... |
|
|
Novel cyclization chemistry especially suited for biologically derived, unprotected peptides.
Int. J. Pept. Protein Res. 39(6) , 533-9, (1992) A novel method is described for the cyclization of peptides--or segments of polypeptides--which requires a free N-terminal alpha-amino group and a distal amino acid residue containing a nucleophilic side chain. The reaction is conducted in two steps, both in ... |