4-乙氧基苯酚
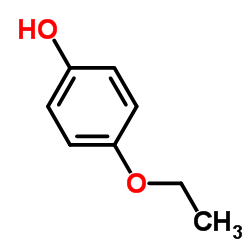
4-乙氧基苯酚结构式

|
常用名 | 4-乙氧基苯酚 | 英文名 | 4-Ethoxyphenol |
|---|---|---|---|---|
| CAS号 | 622-62-8 | 分子量 | 138.164 | |
| 密度 | 1.1±0.1 g/cm3 | 沸点 | 246.4±13.0 °C at 760 mmHg | |
| 分子式 | C8H10O2 | 熔点 | 64-67 °C | |
| MSDS | 中文版 美版 | 闪点 | 124.0±4.8 °C | |
| 符号 |

GHS07 |
信号词 | Warning |
|
Convenient QSAR model for predicting the complexation of structurally diverse compounds with β-cyclodextrins
Bioorg. Med. Chem. 17 , 896-904, (2009) This paper reports a QSAR study for predicting the complexation of a large and heterogeneous variety of substances (233 organic compounds) with beta-cyclodextrins (beta-CDs). Several different theoretical molecular descriptors, calculated solely from the mole... |
|
|
Cellular apoptosis and cytotoxicity of phenolic compounds: a quantitative structure-activity relationship study.
J. Med. Chem. 48 , 7234-42, (2005) In this comprehensive study on the caspase-mediated apoptosis-inducing effect of 51 substituted phenols in a murine leukemia cell line (L1210), we determined the concentrations needed to induce caspase activity by 50% (I50) and utilized these data to develop ... |
|
|
Microperoxidase/H2O2-mediated alkoxylating dehalogenation of halophenol derivatives in alcoholic media.
Proc. Natl. Acad. Sci. U. S. A. 94(9) , 4295-9, (1997) The results of this study report the H2O2-driven microperoxidase-8 (MP8)-catalyzed dehalogenation of halophenols such as 4-fluorophenol, 4-chlorophenol, 4-bromophenol, and 2-fluorophenol in alcoholic solvents. In methanol, the conversion of the para-halopheno... |
|
|
Kinetic characterization of the substrate specificity and mechanism of mushroom tyrosinase.
Eur. J. Biochem. 267(5) , 1270-9, (2000) This paper reports a quantitative study of the effect of ring substituents in the 1-position of the aromatic ring on the rate of monophenol hydroxylation and o-diphenol oxidation catalyzed by tyrosinase. A possible correlation between the electron density of ... |