1,2-亚苯基-双-马来酰亚胺
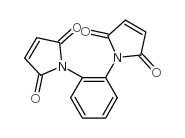
1,2-亚苯基-双-马来酰亚胺结构式

|
常用名 | 1,2-亚苯基-双-马来酰亚胺 | 英文名 | 1H-Pyrrole-2,5-dione,1,1'-(1,2-phenylene)bis |
|---|---|---|---|---|
| CAS号 | 13118-04-2 | 分子量 | 268.22400 | |
| 密度 | 1.567g/cm3 | 沸点 | 459.7ºC at 760mmHg | |
| 分子式 | C14H8N2O4 | 熔点 | 245-247ºC (dec.)(lit.) | |
| MSDS | 中文版 美版 | 闪点 | 223.7ºC |
|
Use of maleimide-thiol coupling chemistry for efficient syntheses of oligonucleotide-enzyme conjugate hybridization probes.
Bioconjug. Chem. 1 , 71-76, (1990) Two general methods which exploit the reactivity of sulfhydryl groups toward maleimides are described for the synthesis of oligonucleotide-enzyme conjugates for use as nonradioisotopic hybridization probes. In the first approach, 6-maleimidohexanoic acid succ... |
|
|
Subunit associations of (Na+ + K+)-dependent adenosine triphosphatase. Chemical cross-linking studies.
J. Biol. Chem. 258(16) , 9878-85, (1983) Cross-linking reactions of the alpha- and beta-subunits of the purified membrane-bound enzyme with several reagents were studied. In the presence of 1,5-difluoro-2,4-dinitrobenzene, formation of a cross-linked alpha, beta-dimer was affected specifically by K+... |
|
|
Helix packing in the lactose permease of Escherichia coli determined by site-directed thiol cross-linking: helix I is close to helices V and XI.
Biochemistry 38(10) , 3120-6, (1999) Coexpression of lacY gene fragments encoding the first two transmembrane domains and the remaining 10 transmembrane domains complement in the membrane and catalyze active lactose transport [Wrubel, W., Stochaj, U., et al. (1990) J. Bacteriol. 172, 5374-5381].... |
|
|
Transmembrane helix tilting and ligand-induced conformational changes in the lactose permease determined by site-directed chemical crosslinking in situ.
J. Mol. Biol. 282(5) , 959-67, (1998) The N-terminal six transmenbrane helices (N6) and the C-terminal six transmembrane helices (C6) of the lactose permease of Escherichia coli, each with a Cys residue, were co-expressed independently, and crosslinking was studied. Proximity of paired Cys residu... |
|
|
Fluctuation of the first loop facing the matrix of the mitochondrial ADP/ATP carrier deduced from intermolecular cross-linking of Cys56 residues by bifunctional dimaleimides.
Biochemistry 38(3) , 1050-6, (1999) The effects of six thiol-specific cross-linker dimaleimides, in which the distance of the two maleimide groups ranged from 7.7 to 16. 8 A, on bovine heart mitochondria were studied at pH 7.2 and 0 degrees C. None of the dimaleimides affected mitochondrial pro... |
|
|
Tilting of helix I and ligand-induced changes in the lactose permease determined by site-directed chemical cross-linking in situ.
Biochemistry 37(45) , 15785-90, (1998) The N-terminal six transmenbrane helices (N6) and the C-terminal six transmembrane helices (C6) of lactose permease, each with a single Cys residue, were co-expressed, and cross-linking was studied. The proximity of paired Cys residues in helices I (positions... |
|
|
Two ATP synthases can be linked through subunits i in the inner mitochondrial membrane of Saccharomyces cerevisiae.
Biochemistry 41(33) , 10390-6, (2002) Cross-linking experiments showed that the supernumerary subunit i is close to the interface between two ATP synthases. These data were used to demonstrate the presence of ATP synthase dimers in the inner mitochondrial membrane of Saccharomyces cerevisiae. A c... |
|
|
Site-directed chemical cross-linking demonstrates that helix IV is close to helices VII and XI in the lactose permease.
Biochemistry 38(6) , 1715-20, (1999) The N-terminal six transmenbrane helices (N6) and the C-terminal six transmembrane helices (C6) of the lactose permease, each containing a single-Cys residue, were coexpressed, and proximity was studied. Paired Cys residues in helices IV (positions 114, 116, ... |
|
|
The conformation of cross-linked actin.S-1 in the presence and absence of ATP.
J. Biol. Chem. 262(13) , 6128-34, (1987) Electron microscopy studies have shown that the structure of the complex of myosin subfragment 1 (S-1) cross-linked to actin with 1-ethyl-3-[3-(dimethyl-amino) propyl] carbodiimide is very different in the presence and absence of ATP (Craig, R., Greene, L. E.... |
|
|
Topological and functional study of subunit h of the F1Fo ATP synthase complex in yeast Saccharomyces cerevisiae.
Biochemistry 42(41) , 12038-49, (2003) Subunit h, a 92-residue-long, hydrophilic, acidic protein, is a component of the yeast mitochondrial F1Fo ATP synthase. This subunit, homologous to the mammalian factor F6, is essential for the correct assembly and/or functioning of this enzyme since yeast ce... |