| 结构式 | 名称/CAS号 | 全部文献 |
|---|---|---|
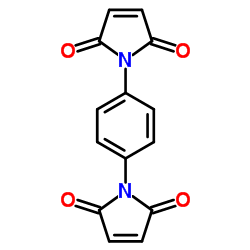 |
N,N'-1,4-亚苯基二马来酰亚胺
CAS:3278-31-7 |
|
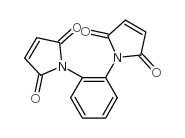 |
1,2-亚苯基-双-马来酰亚胺
CAS:13118-04-2 |