二氯二茂锆
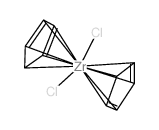
二氯二茂锆结构式

|
常用名 | 二氯二茂锆 | 英文名 | Bis(cyclopentadienyl)zirconium dichloride |
|---|---|---|---|---|
| CAS号 | 1291-32-3 | 分子量 | 284.25300 | |
| 密度 | N/A | 沸点 | 41.5ºC at 760 mmHg | |
| 分子式 | C10H2Cl2Zr | 熔点 | 242-245ºC | |
| MSDS | 中文版 美版 | 闪点 | 124-125°C/15mm | |
| 符号 |

GHS07 |
信号词 | Warning |
|
Unusual temperature effects in propene polymerization using stereorigid zirconocene catalysts.
ChemPhysChem 6(9) , 1929-33, (2005) Free Car-Parrinello molecular dynamics (CPMD) simulations of four diastereomers of the zirconium-propene complexes [{iPr(3-iPr-CpFlu)}ZriBu(C3H6)]+ (Cp=cyclopentadienyl; Flu=fluorenyl) provide valuable insight into the mechanism and stereocontrol of propene p... |
|
|
Enantioselective route from carbohydrates to cyclooctane polyols.
Org. Lett. 7(3) , 511-3, (2005) [reaction: see text] A synthetic route to select cyclooctane-1,2,3-triols and 1,2,3,4,5-pentaols has been defined. The starting materials are d-glucose or d-arabinose, and the key steps consist of a zirconocene-promoted ring contraction, a [3,3] sigmatropic r... |
|
|
Functionalised cyclopentadienyl zirconium compounds as potential anticancer drugs.
Dalton Trans. (39) , 5293-5, (2008) New neutral and ionic functionalised zirconocene dichloride compounds have been isolated and characterised. The ionic zirconocene exhibits excellent cytotoxicity against a range of human tumour cell lines, which represents the first active anticancer zirconoc... |
|
|
Formal total synthesis of (+/-)-estrone and zirconocene-promoted cyclization of 2-fluoro-1,7-octadienes and ru-catalyzed ring closing metathesis.
J. Org. Chem. 73(16) , 6202-6, (2008) A new and diastereoselective method for the synthesis of the estrone skeleton from a substituted styrene based on sequential 3-fold use of Cp 2ZrBu 2 (oxidative addition-alkylation and two cyclization-alkylation sequences) and a ruthenium complex catalyzed RC... |
|
|
In pursuit of pestalotiopsin a via zirconocene-mediated ring contraction.
Org. Lett. 8(11) , 2429-31, (2006) [reaction: see text] An asymmetric route from the epimeric beta-hydroxy esters 4 and 5 to the densely functionalized (+)-10 and (-)-10, respectively, is described. Either cyclobutanol can be made available as the predominant product. The levorotatory antipode... |
|
|
A selective synthesis of hydroxyborate anions as novel anchors for zirconocene catalysts.
Dalton Trans. (21) , 2866-70, (2008) A new family of hydroxytris(pentafluorophenyl)borate anions [B(C6F5)3OH](-) associated with organic and aprotic cations c+ (imidazolium, pyrrolidinium and phosphonium) has been prepared by a general one-pot synthesis that implies the chloride borate analogues... |
|
|
Double carbonylation of zirconocene-alkyne complexes.
Chem. Commun. (Camb.) (19) , 2495-6, (2005) Zirconocene-alkyne complexes prepared from Cp2ZrBu2, phosphines and alkynes reacted with CO to give double carbonylation products, 4-hydroxycyclobuten-1-one derivatives after hydrolysis. |
|
|
Zirconocene-mediated synthesis of cyclobutabenzenes.
Org. Lett. 10(20) , 4469-71, (2008) A new, mild, one-pot method for the synthesis of cyclobutabenzenes by the zirconium-promoted cross-coupling reaction of aryllithium compounds and alkenyl bromides is reported. Formation of an arynezirconocene complex and its regioselective coupling with an al... |
|
|
Medium-sized carbocycles by a zirconocene-catalyzed tandem formal ring expansion-magnesation reaction of alkenyl-substituted cyclic enol ethers.
Org. Lett. 9(16) , 3081-4, (2007) A new regio- and stereoselective zirconocene-catalyzed reaction for the synthesis of medium-sized rings is described. The global reaction supposes a formal ring expansion of a cyclic enol ether to give a functionalized carbocycle. |
|
|
Tandem insertion of halocarbenoids and lithium acetylides into zirconacycles: a novel rearrangement to zirconium alkenylidenates by β-addition to an alkynyl zirconocene.
Chemistry 17(17) , 4896-904, (2011) Tandem insertion of 1,1-dihalo-1-lithio species (halocarbenoids) and lithium alkynides into zirconacyclopentenes and zirconcyclopentanes affords carbocyclic products in high yields via an unusual rearrangement that probably involves addition of an organolithi... |