聚左旋脯氨酸
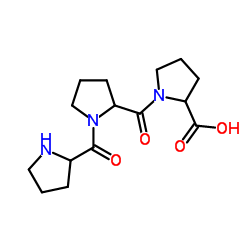
聚左旋脯氨酸结构式

|
常用名 | 聚左旋脯氨酸 | 英文名 | N-Fmoc-1,6-hexanediamine hydrobromide |
|---|---|---|---|---|
| CAS号 | 25191-13-3 | 分子量 | 309.361 | |
| 密度 | 1.3±0.1 g/cm3 | 沸点 | 602.0±55.0 °C at 760 mmHg | |
| 分子式 | C15H23N3O4 | 熔点 | N/A | |
| MSDS | 美版 | 闪点 | 317.9±31.5 °C |
|
Well-defined homopolypeptides, copolypeptides, and hybrids of poly(l-proline).
Biomacromolecules 12 , 2396-2406, (2011) l-Proline is the only, out of 20 essential, amino acid that contains a cyclized substituted α-amino group (is formally an imino acid), which restricts its conformational shape. The synthesis of well-defined homo- and copolymers of l-proline has been plagued e... |
|
|
Polyproline fold-In imparting kinetic stability to an alkaline serine endopeptidase.
Biochim. Biophys. Acta 1834(3) , 708-16, (2013) Polyproline II (PPII) fold, an unusual structural element was detected in the serine protease from Nocardiopsis sp. NCIM 5124 (NprotI) based on far UV circular dichroism spectrum, structural transitions of the enzyme in presence of GdnHCl and a distinct isodi... |
|
|
The Folding process of Human Profilin-1, a novel protein associated with familial amyotrophic lateral sclerosis.
Sci. Rep. 5 , 12332, (2015) Human profilin-1 is a novel protein associated with a recently discovered form of familial amyotrophic lateral sclerosis. This urges the characterization of possible conformational states, different from the fully folded state, potentially able to initiate se... |
|
|
Interactions between homopolypeptides and lightly cross-linked microgels.
Langmuir 25 , 522-528, (2009) The relative importance of electrostatic and nonelectrostatic interactions in peptide-microgel systems was evaluated by micromanipulator-assisted light microscopy, confocal microscopy, and circular dichroism. For this purpose, the interaction of various homop... |
|
|
n→π* interactions in poly(lactic acid) suggest a role in protein folding.
Chem. Commun. (Camb.) 49(70) , 7699-701, (2013) Poly(lactic acid) (PLA) is a versatile synthetic polyester. We noted that this depsipeptide analog of polyalanine has a helical structure that resembles a polyproline II helix. Using natural bond orbital analysis, we find that n→π* interactions between sequen... |
|
|
Photoinitiated intramolecular diradical cross-linking of polyproline peptides in the gas phase.
Phys. Chem. Chem. Phys. 14(47) , 16243-9, (2012) Polyproline is a fascinating polymer with interesting structural properties that have been studied in both solution and the gas phase. Herein, a method capable of measuring structural dynamics over long timescales is developed and applied to examination of po... |
|
|
The proline-rich tetramerization peptides in equine serum butyrylcholinesterase.
FEBS J. 279(20) , 3844-58, (2012) Soluble, tetrameric, plasma butyrylcholinesterase from horse has previously been shown to include a non-covalently attached polyproline peptide in its structure. The polyproline peptide matched the polyproline-rich region of human lamellipodin. Our goal was t... |
|
|
Evolutionary conservation of the polyproline II conformation surrounding intrinsically disordered phosphorylation sites.
Protein Sci. 22(4) , 405-17, (2013) Intrinsically disordered (ID) proteins function in the absence of a unique stable structure and appear to challenge the classic structure-function paradigm. The extent to which ID proteins take advantage of subtle conformational biases to perform functions, a... |
|
|
Evolution of the hepatitis E virus polyproline region: order from disorder.
J. Virol. 86(18) , 10186-93, (2012) The hepatitis E virus (HEV) polyproline region (PPR) is an intrinsically unstructured region (IDR). This relaxed structure allows IDRs, which are implicated in the regulation of transcription and translation, to bind multiple ligands. Originally the nucleotid... |
|
|
Glycine rescue of β-sheets from cis-proline.
J. Am. Chem. Soc. 134(40) , 16536-9, (2012) Proline is incompatible with ideal β-sheet geometry, and the incompatibility gets magnified when Pro assumes the cis peptidyl-prolyl conformation. We show that Gly appears with high propensity at pre-cisPro positions in β-sheets and rescues the β-sheet from s... |