| 结构式 | 名称/CAS号 | 全部文献 |
|---|---|---|
 |
水
CAS:7732-18-5 |
|
 |
溴化氰活化的琼脂糖
CAS:68987-32-6 |
|
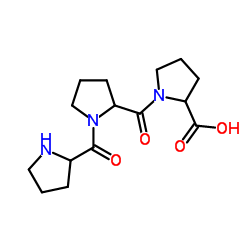 |
聚左旋脯氨酸
CAS:25191-13-3 |