L-脯氨醇
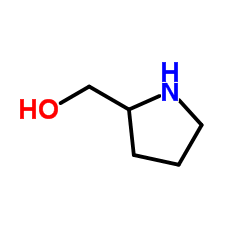
L-脯氨醇结构式

|
常用名 | L-脯氨醇 | 英文名 | L-Pro-ol |
|---|---|---|---|---|
| CAS号 | 23356-96-9 | 分子量 | 101.147 | |
| 密度 | 1.0±0.1 g/cm3 | 沸点 | 211.0±0.0 °C at 760 mmHg | |
| 分子式 | C5H11NO | 熔点 | N/A | |
| MSDS | 美版 | 闪点 | 86.1±0.0 °C | |
| 符号 |

GHS07 |
信号词 | Warning |
|
Three-dimensional quantitative structure-activity relationship analyses of substrates of the human proton-coupled amino acid transporter 1 (hPAT1).
Bioorg. Med. Chem. 19 , 6409-18, (2011) The proton-coupled amino acid transporter hPAT1 has recently gained much interest due to its ability to transport small drugs thereby allowing their oral administration. A three-dimensional quantitative structure-activity relationship (3D QSAR) study has been... |
|
|
Chiral Diphenylprolinol TES ether promoted conjugate addition-aldol-dehydration reactions between alpha,beta-unsaturated aldehydes and 2-N-protected amino benzaldehydes.
Org. Lett. 9(6) , 965-8, (2007) A conjugate addition-aldol-dehydration reaction of alpha,beta-unsaturated aldehydes with 2-N-protected amino benzaldehydes has been developed. The process is promoted by (S)-diphenylprolinol TES ether to afford synthetically useful 1,2-dihydroquinolines in hi... |
|
|
Asymmetric alkynylation of aldehydes with propiolates without high reagent loading and any additives.
Org. Biomol. Chem. 9(12) , 4425-8, (2011) The asymmetric alkynylation of aliphatic and aromatic aldehydes with propiolates was mediated by dialkylzinc and a novel prolinol catalyst without high reagent loading and any additives, such as Ti(Oi-Pr)(4), to give the corresponding γ-hydroxy-α,β-acetylenic... |
|
|
CBS reductions with a fluorous prolinol immobilized in a hydrofluoroether solvent.
Org. Lett. 10(5) , 749-52, (2008) A fluorous prolinol precatalyst bearing only 34 fluorine atoms has been immobilized in the hydrofluoroether solvent HFE-7500. The CBS reduction of acetophenone proceeded rapidly, in high yield and in high ee in the absence of any organic solvent. The organic ... |
|
|
Access to optically active 3-azido- and 3-aminopiperidine derivatives by enantioselective ring expansion of prolinols.
Org. Lett. 13(16) , 4442-5, (2011) The activation of N-alkyl prolinols by XtalFluor E allowed the formation of an aziridinium intermediate that can react with tetrabutylammonium azide (nBu(4)NN(3)) to produce 3-azidopiperidines and/or 2-(azidomethyl)pyrrolidines, in a ratio up to 100/0. These ... |
|
|
Efficient proline and prolinol ether mediated 3-component synthesis of 3- and 3,4-substituted chromenone derivatives.
Org. Biomol. Chem. 10(30) , 6201-10, (2012) A highly efficient route for the synthesis of valuable 3,4-substituted chromenone derivatives by the reaction of 1,3-diketones with aldehydes in the presence of l-proline was developed. The reactions take advantage of readily available starting materials and ... |
|
|
Organocatalytic asymmetric epoxidation and tandem epoxidation/Passerini reaction under eco-friendly reaction conditions.
Org. Biomol. Chem. 10(38) , 7681-4, (2012) An eco-friendly synthesis of highly functionalized epoxides and their incorporation into an organocatalytic multicomponent approach are reported. For this, a modified class of diarylprolinol silyl ethers was designed to enable high catalytic activity in an en... |
|
|
An organocascade kinetic resolution.
Org. Lett. 13(24) , 6460-3, (2011) Products of a novel iminium-catalyzed oxa-Michael addition undergo a kinetic resolution by a subsequent enamine-catalyzed intermolecular reaction. This is a rare example of kinetic resolution by enamine catalysis and the first organocascade kinetic resolution... |
|
|
Selective methodologies for the synthesis of biologically active piperidinic compounds.
Chem. Rec. 5(2) , 70-80, (2005) The synthesis of optically active substituted piperidines has been achieved by using four different methodologies. The first one is an intramolecular nucleophilic displacement of activated alcohol moieties that was used to build up the piperidine ring of (-)-... |
|
|
Amination of N-aryl prolinol via ring expansion and contraction: application to the chiral ligand for the catalytic asymmetric reaction.
J. Org. Chem. 70(5) , 1937-40, (2005) Chiral diaminophosphines 4 were prepared from (S)-prolinol-derived aminophosphine oxide 5 by bromination with ring expansion followed by amination with ring contraction and reduction, using trichlorosilane. In the presence of 4 as a ligand, palladium-catalyze... |