亚硝酸丁酯
一般危化品
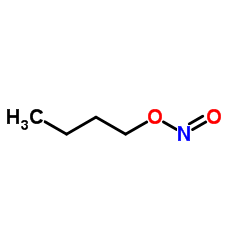
亚硝酸丁酯结构式
|
常用名 | 亚硝酸丁酯 | 英文名 | Butyl nitrite |
|---|---|---|---|---|
| CAS号 | 544-16-1 | 分子量 | 103.120 | |
| 密度 | 1.0±0.1 g/cm3 | 沸点 | 78.8±3.0 °C at 760 mmHg | |
| 分子式 | C4H9NO2 | 熔点 | N/A | |
| MSDS | 中文版 美版 | 闪点 | -13.3±0.0 °C | |
| 符号 |


GHS02, GHS06 |
信号词 | Danger |
|
Chemoselective nitration of aromatic sulfonamides with tert-butyl nitrite.
Chem. Commun. (Camb.) 49(5) , 514-6, (2013) A methodology for the efficient conversion of aromatic sulfonamides into their mono-nitro derivatives using tert-butyl nitrite is reported. The reaction exhibits a high degree of chemoselectivity for sulfonamide functionalized aryl systems, even in the presen... |
|
|
Post-translational modification in the gas phase: mechanism of cysteine S-nitrosylation via ion-molecule reactions.
Rapid Commun. Mass Spectrom. 25(21) , 3216-22, (2011) The gas-phase mechanism of S-nitrosylation of thiols was studied in a quadrupole ion trap mass spectrometer. This was done via ion-molecule reactions of protonated cysteine and many of its derivatives and other thiol ions with neutral tert-butyl nitrite or ni... |
|
|
Acute effects of some volatile nitrites on motor performance and lethality in mice.
Neurobehav. Toxicol. Teratol. 8(2) , 139-42, (1986) Mice were examined for effects on lethality and motor performance on an inverted screen test following inhalation exposure to isoamyl, n-butyl and isobutyl nitrite. All three nitrites produced concentration-related effects on both measures, with EC50s for mot... |
|
|
Toxicity, immunosuppressive effects and carcinogenic potential of volatile nitrites: possible relationship to Kaposi's sarcoma.
Pharmacotherapy 4(5) , 284-91, (1984) Volatile nitrite in the form of amyl nitrite was used for 100 years for the treatment of angina pectoris. In spite of recognized toxicity, its use in this form was considered safe. During the 1960s prescriptions were not required for purchase of amyl nitrite ... |
|
|
Disruption of auditory function by acute administration of a “room odorizer” containing butyl nitrite in rats
Fundam. Appl. Toxicol. 12(1) , 56-61, (1989) Butyl nitrite is the predominant and presumed active ingredient in a variety of commercial preparations sold as “room odorizers.” These compounds have significant abuse potential, giving the user the sensation of a “rush,” which may be related to their intens... |
|
|
Subchronic toxicology of butyl nitrites in mice by inhalation.
J. Appl. Toxicol. 5(3) , 134-9, (1985) Mice were exposed by inhalation to n-butyl, iso-butyl sec-butyl or tert-butyl nitrite in a dynamic airflow chamber 7 h per day for 60 days at concentrations that caused less than 20% fatalities. Under these conditions, body-weight gain was depressed over the ... |
|
|
Nitric oxide-releasing ruthenium nanoparticles.
Chem. Commun. (Camb.) 47(38) , 10776-8, (2011) Nitric oxide-releasing ruthenium nanoparticles were synthesized by the reaction of alkanethiolate-protected ruthenium nanoparticles with tert-butyl nitrite ((t)BuONO), and their water-soluble derivatives are able to deliver NO to proteins such as reduced myog... |
|
|
Vibrational analysis of n-butyl, isobutyl, sec-butyl and tert-butyl nitrite.
Spectrochim. Acta. A. Mol. Biomol. Spectrosc. 67(1) , 178-87, (2007) Raman and infrared spectra of n-butyl, isobutyl, sec-butyl and tert-butyl nitrite are reported. Density functional theory and Møeller-Plesset calculations with 6-31G* and 6-311G* basis sets were used to determine ground state molecular geometries and vibratio... |
|
|
Uptake of inhaled n-butyl nitrite and in vivo transformation in rats.
J. Pharm. Sci. 74(7) , 780-2, (1985) The uptake of butyl nitrite by rats (500 g, one rat/chamber) was determined over a 5-min exposure period. About 44% of the starting amount (771-3855 ppm) of n-butyl nitrite was consumed in 5 min. Three rats per exposure concentration were individually studied... |
|
|
Kinetics of n-butoxy and 2-pentoxy isomerization and detection of primary products by infrared cavity ringdown spectroscopy.
J. Phys. Chem. A 116(24) , 6327-40, (2012) The primary products of n-butoxy and 2-pentoxy isomerization in the presence and absence of O(2) have been detected using pulsed laser photolysis-cavity ringdown spectroscopy (PLP-CRDS). Alkoxy radicals n-butoxy and 2-pentoxy were generated by photolysis of a... |