苏达灭
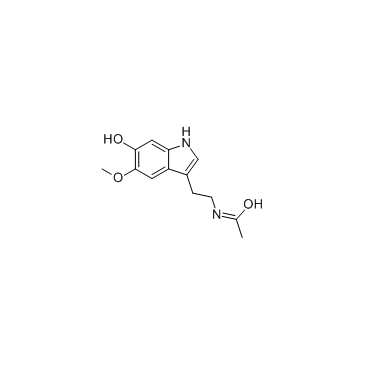
苏达灭结构式

|
常用名 | 苏达灭 | 英文名 | 6-Hydroxymelatonin |
|---|---|---|---|---|
| CAS号 | 2208-41-5 | 分子量 | 248.27800 | |
| 密度 | 1.266g/cm3 | 沸点 | 564.7ºC at 760mmHg | |
| 分子式 | C13H16N2O3 | 熔点 | 172-175ºC | |
| MSDS | 中文版 美版 | 闪点 | 295.3ºC | |
| 符号 |


GHS07, GHS08 |
信号词 | Warning |
|
Melatonin and its metabolites accumulate in the human epidermis in vivo and inhibit proliferation and tyrosinase activity in epidermal melanocytes in vitro.
Mol. Cell. Endocrinol. 404 , 1-8, (2015) Melatonin and its metabolites including 6-hydroxymelatonin (6(OH)M), N(1)-acetyl-N(2)-formyl-5-methoxykynuramine (AFMK) and 5-methoxytryptamine (5MT) are endogenously produced in human epidermis. This production depends on race, gender and age. The highest me... |
|
|
Predominance of 2-hydroxymelatonin over melatonin in plants.
J. Pineal Res. 59 , 448-54, (2015) The cloning of the gene encoding melatonin 2-hydroxylase (M2H), which is responsible for the synthesis of 2-hydroxymelatonin, has expanded the study of melatonin metabolism in plants. Kinetic analysis of M2H enzymatic activity demonstrated that the catalytic ... |
|
|
Molecular cloning of melatonin 2-hydroxylase responsible for 2-hydroxymelatonin production in rice (Oryza sativa).
J. Pineal Res. 58(3) , 343-51, (2015) Although melatonin biosynthetic genes from plants have been cloned, the melatonin catabolism mechanisms remain unclear. To clone the genes responsible for melatonin metabolism, we ectopically expressed 35 full-length cDNAs of rice 2-oxoglutarate-dependent dio... |
|
|
Sulfation of melatonin: enzymatic characterization, differences of organs, species and genders, and bioactivity variation.
Biochem. Pharmacol. 94(4) , 282-96, (2015) Exogenous melatonin (Mel) is widely used in clinic for multiple therapeutic purposes. In metabolism pathways of Mel, 6-hydroxymelatonin-sulfate (S-O-Mel) and N-acetylserotonin sulfate (S-NAS) are the most abundant metabolites account for over 90% of total Mel... |
|
|
Metabolism of melatonin by cytochrome P450s in rat liver mitochondria and microsomes.
J. Pineal Res. 45(4) , 515-23, (2008) In the present study we provide direct evidence for the involvement of rat microsomal cytochrome P450s in melatonin O-demethylation and hydroxylation at two different positions: 2 and 6, as well as generation of N(1)-acetyl-N(2)-formyl-5-methoxy-kynuramine (A... |
|
|
Comparison of the pharmacological characteristics of 2-[125I]iodomelatonin binding sites in the lung, spleen, brain and kidney of chicken.
Biol. Signals 4 , 311, (1995) We have compared the pharmacological characteristics of 2-[125I]iodomelatonin binding to crude membrane preparations of the lung, spleen, brain and kidney of chicken. Saturation studies indicated significant differences (p < 0.05) in the equilibrium dissociat... |
|
|
Metabolism of melatonin and biological activity of intermediates of melatoninergic pathway in human skin cells.
FASEB J. 27(7) , 2742-55, (2013) Indolic and kynuric pathways of skin melatonin metabolism were monitored by liquid chromatography mass spectrometry in human keratinocytes, melanocytes, dermal fibroblasts, and melanoma cells. Production of 6-hydroxymelatonin [6(OH)M], N(1)-acetyl-N(2)-formyl... |
|
|
Plasma melatonin and urinary 6-hydroxymelatonin levels in patients with pulmonary tuberculosis.
Inflammation 35(4) , 1429-34, (2012) Tuberculosis (TB) is the second most frequent cause of death in the world, after AIDS. Delay in diagnosing TB is an important worldwide problem. It seriously threatens public health. Cell-mediated immune responses play an important role in the pathogenesis of... |
|
|
The excretion of 6-hydroxymelatonin sulfate in healthy young men exposed to electromagnetic fields emitted by cellular phone -- an experimental study.
Neuro Endocrinol. Lett. 23 Suppl 1 , 88-91, (2002) It is quite likely that non-visible electromagnetic fields (EMF) may affect melatonin production. Some studies confirmed this hypothesis and showed that extremely low EMF altered pineal function in animals and humans. Thus, it is reasonable to suppose that EM... |
|
|
Melatonin as an effective protector against doxorubicin-induced cardiotoxicity.
Am. J. Physiol. Heart Circ. Physiol. 283(1) , H254-63, (2002) The present study was designed to explore the protective effects of melatonin and its analogs, 6-hydroxymelatonin and 8-methoxy-2-propionamidotetralin, on the survival of doxorubicin-treated mice and on doxorubicin-induced cardiac dysfunction, ultrastructural... |