琥珀酸多西拉敏
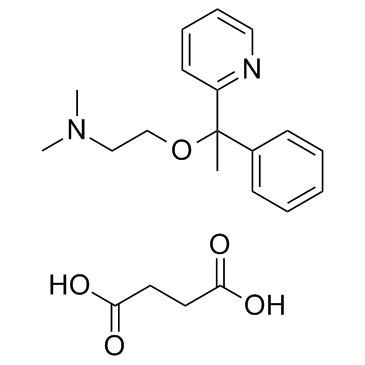
琥珀酸多西拉敏结构式

|
常用名 | 琥珀酸多西拉敏 | 英文名 | DOXYLAMINE SUCCINATE |
|---|---|---|---|---|
| CAS号 | 562-10-7 | 分子量 | 388.457 | |
| 密度 | 1.043g/cm3 | 沸点 | 364.9ºC at 760 mmHg | |
| 分子式 | C21H28N2O5 | 熔点 | 103 - 108ºC | |
| MSDS | 中文版 美版 | 闪点 | 174.5ºC | |
| 符号 |

GHS07 |
信号词 | Warning |
|
Use of In Vitro Morphogenesis of Mouse Embryoid Bodies to Assess Developmental Toxicity of Therapeutic Drugs Contraindicated in Pregnancy.
Toxicol. Sci. 149 , 15-30, (2016) In utero exposure to certain chemicals can impair embryo development, causing embryonic death, growth retardation, or severe birth defects. Establishment of effective in vitro tests is crucial for identifying developmental toxicants and for reducing the finan... |
|
|
[Nontraumatic rhabdomyolysis due to oral poisoning by doxylamine succinate].
Med. Clin. (Barc.) 108(9) , 356, (1997)
|
|
|
Safety assessment of OTC drugs: doxylamine succinate.
Arch. Toxicol. Suppl. 17 , 326-40, (1995)
|
|
|
Dose-response trend tests for tumorigenesis, adjusted for body weight.
Toxicol. Sci. 49(2) , 318-23, (1999) Several studies have demonstrated a relationship between rodent body weight and tumor incidence for some tissue/organ sites. It is not uncommon for a chemical tested for carcinogenicity to also affect body weight. In such cases, comparisons of tumor incidence... |
|
|
Desorption chemical ionization and fast atom bombardment mass spectrometric studies of the glucuronide metabolites of doxylamine.
Biomed. Environ. Mass Spectrom. 13(11) , 627-32, (1986) Three glucuronide metabolites of doxylamine succinate were collected in a single fraction using high-performance liquid chromatography (HPLC) from the urine of dosed male Fischer 344 rats. The metabolites were then separated using an additional HPLC step into... |
|
|
Metabolism of doxylamine succinate in Fischer 344 rats. Part II: Nonconjugated urinary and fecal metabolites.
J. Anal. Toxicol. 11(3) , 113-21, (1987) Elimination and metabolic profiles of doxylamine and its nonconjugated metabolites were determined after the oral administration of [14C]-doxylamine succinate (13.3 mg/kg and 133 mg/kg doses) to male and female Fischer 344 rats. Total urine and fecal recovery... |
|
|
Mutagenicity testing of doxylamine succinate, an antinauseant drug.
Toxicol. Lett. 49(1) , 79-86, (1989) Doxylamine succinate (DA), a compound which was formerly used as an antinauseant during pregnancy, showed no substantial mutagenicity in mouse embryos following transplacental exposure. A small dose-dependent induction of chromosomal aberrations was found in ... |
|
|
Severe rhabdomyolysis after doxylamine overdose.
Postgrad. Med. 93(8) , 227-9, 232, (1993) Clinicians should be aware of the complications of rhabdomyolysis in patients who ingest doxylamine succinate and other over-the-counter antihistamines. The easy availability of these substances increases the potential not only for intentional overdose by adu... |
|
|
Separation of cold medicine ingredients using a precise MEKC method at elevated pH.
Electrophoresis 28(11) , 1779-87, (2007) An MEKC method was developed in order to separate a cold medicine formulation containing acetaminophen, ephedrine sulfate, doxylamine succinate, and dextromethorphan hydrobromide as active pharmaceutical ingredients. Because of their similar physical and chem... |
|
|
Doxylamine and diphenhydramine pharmacokinetics in women on low-dose estrogen oral contraceptives.
J. Clin. Pharmacol. 29(3) , 257-60, (1989) Thirteen women chronically using low-dose estrogen-containing oral contraceptives (50 micrograms or less of ethinyl estradiol or its equivalent for a minimum of 3 months) and 12 age-matched drug-free control women received a single 25 mg oral dose of doxylami... |