Tetracene
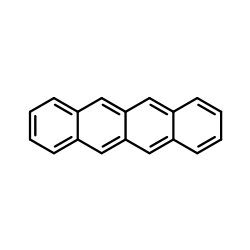
Tetracene structure
|
Common Name | Tetracene | ||
|---|---|---|---|---|
| CAS Number | 92-24-0 | Molecular Weight | 228.288 | |
| Density | 1.2±0.1 g/cm3 | Boiling Point | 436.7±12.0 °C at 760 mmHg | |
| Molecular Formula | C18H12 | Melting Point | >300 °C(lit.) | |
| MSDS | Chinese USA | Flash Point | 209.1±13.7 °C | |
| Name | tetracene |
|---|---|
| Synonym | More Synonyms |
| Density | 1.2±0.1 g/cm3 |
|---|---|
| Boiling Point | 436.7±12.0 °C at 760 mmHg |
| Melting Point | >300 °C(lit.) |
| Molecular Formula | C18H12 |
| Molecular Weight | 228.288 |
| Flash Point | 209.1±13.7 °C |
| Exact Mass | 228.093903 |
| LogP | 5.91 |
| Vapour Pressure | 0.0±0.5 mmHg at 25°C |
| Index of Refraction | 1.771 |
| InChIKey | IFLREYGFSNHWGE-UHFFFAOYSA-N |
| SMILES | c1ccc2cc3cc4ccccc4cc3cc2c1 |
| Stability | Stable. Incompatible with strong oxidizing agents. |
CHEMICAL IDENTIFICATION
HEALTH HAZARD DATAACUTE TOXICITY DATAMUTATION DATA
|
| Personal Protective Equipment | Eyeshields;Gloves;type N95 (US);type P1 (EN143) respirator filter |
|---|---|
| Hazard Codes | N: Dangerous for the environment; |
| Risk Phrases | R50/53 |
| Safety Phrases | S45-S36/37 |
| RIDADR | UN 3077 9/PG 3 |
| WGK Germany | 3 |
| RTECS | QI7605000 |
| HS Code | 2902909090 |
| Precursor 6 | |
|---|---|
| DownStream 8 | |
| HS Code | 2902909090 |
|---|---|
| Summary | 2902909090 other aromatic hydrocarbons。Supervision conditions:None。VAT:17.0%。Tax rebate rate:9.0%。MFN tariff:2.0%。General tariff:30.0% |
|
QSPR modeling of octanol/water partition coefficient for vitamins by optimal descriptors calculated with SMILES.
Eur. J. Med. Chem. 43 , 714-40, (2008) Simplified molecular input line entry system (SMILES) has been utilized in constructing quantitative structure-property relationships (QSPR) for octanol/water partition coefficient of vitamins and org... |
|
|
First-Principles Prediction of Enthalpies of Formation for Polycyclic Aromatic Hydrocarbons and Derivatives.
J. Phys. Chem. A 119 , 11329-65, (2015) In this article, the first-principles prediction of enthalpies of formation is demonstrated for 669 polycyclic aromatic hydrocarbon (PAH) compounds and a number of related functionalized molecules. It... |
|
|
Large surface relaxation in the organic semiconductor tetracene.
Nat. Commun. 5 , 5400, (2014) Organic crystals are likely to have a large degree of structural relaxation near their surfaces because of the weak inter-molecular interactions. The design of organic field-effect transistors require... |
| Tetracene |
| 2,3-Benzanthracene |
| Naphthacene |
| MFCD00003702 |
| EINECS 202-138-9 |
 CAS#:959-02-4
CAS#:959-02-4 CAS#:3073-99-2
CAS#:3073-99-2 CAS#:85894-23-1
CAS#:85894-23-1 CAS#:13214-70-5
CAS#:13214-70-5 CAS#:256439-90-4
CAS#:256439-90-4 CAS#:96965-79-6
CAS#:96965-79-6 CAS#:68525-40-6
CAS#:68525-40-6 CAS#:2154-56-5
CAS#:2154-56-5 CAS#:193-44-2
CAS#:193-44-2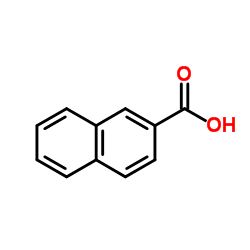 CAS#:93-09-4
CAS#:93-09-4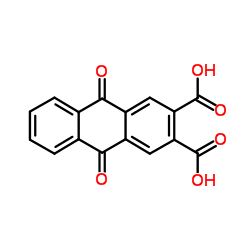 CAS#:27485-15-0
CAS#:27485-15-0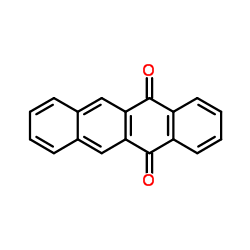 CAS#:1090-13-7
CAS#:1090-13-7![6,13-Dihydro-6,13-[1,2]benzenopentacene structure](https://image.chemsrc.com/caspic/137/16783-54-3.png) CAS#:16783-54-3
CAS#:16783-54-3