Ropivacaine
Modify Date: 2024-01-02 07:15:11
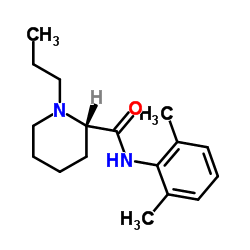
Ropivacaine structure
|
Common Name | Ropivacaine | ||
|---|---|---|---|---|
| CAS Number | 84057-95-4 | Molecular Weight | 274.401 | |
| Density | 1.0±0.1 g/cm3 | Boiling Point | 410.2±45.0 °C at 760 mmHg | |
| Molecular Formula | C17H26N2O | Melting Point | 144 - 146ºC | |
| MSDS | N/A | Flash Point | 201.9±28.7 °C | |
Use of RopivacaineRopivacain is a potent sodium channel blocker and acts as a local anesthetic agent. Ropivacain blocks impulse conduction via reversible inhibition of sodium ion influx in nerve fibrese[1][2]. Ropivacaine is also an inhibitor of K2P (two-pore domain potassium channel) TREK-1 with an IC50 of 402.7 μM in COS-7 cell's membrane[3]. Ropivacaine is used for the research of regional anesthesia and neuropathic pain management[1]. |
| Name | (S)-ropivacaine |
|---|---|
| Synonym | More Synonyms |
| Description | Ropivacain is a potent sodium channel blocker and acts as a local anesthetic agent. Ropivacain blocks impulse conduction via reversible inhibition of sodium ion influx in nerve fibrese[1][2]. Ropivacaine is also an inhibitor of K2P (two-pore domain potassium channel) TREK-1 with an IC50 of 402.7 μM in COS-7 cell's membrane[3]. Ropivacaine is used for the research of regional anesthesia and neuropathic pain management[1]. |
|---|---|
| Related Catalog | |
| Target |
IC50: sodium ion influx[1] IC50: 402.7 μM (TREK-1 in COS-7 cell's membrane)[2] |
| In Vivo | Epidural administration of Ropivacaine effectively blocks neuropathic pain (both mechanical allodynia and heat hyperalgesia) without induction of analgesic tolerance and significantly delays the development of neuropathic pain produced by peripheral nerve injury[1]. Ropivacaine inhibits pressure-induced increases in filtration coefficient (Kf) without affecting pulmonary artery pressure (Ppa), pulmonary capillary pressures (Ppc), and zonal characteristics (ZC)[2]. Ropivacaine prevents pressure-induced lung edema and associated hyperpermeability as evidence by maintaining PaO2, lung wet-to-dry ratio and plasma volume in levels similar to sham rats[2]. Ropivacaine inhibits pressure-induced NO production as evidenced by decreased lung nitro-tyrosine content when compared to hypertensive lungs[2]. Animal Model: Adult Sprague-Dawley rats (300–400g)[1] Dosage: 1 μM Administration: Infusion (added to the perfusate reservoir) Result: Attenuated pressure-dependent increases in filtration coefficient (Kf). |
| References |
| Density | 1.0±0.1 g/cm3 |
|---|---|
| Boiling Point | 410.2±45.0 °C at 760 mmHg |
| Melting Point | 144 - 146ºC |
| Molecular Formula | C17H26N2O |
| Molecular Weight | 274.401 |
| Flash Point | 201.9±28.7 °C |
| Exact Mass | 274.204498 |
| PSA | 32.34000 |
| LogP | 3.11 |
| Vapour Pressure | 0.0±1.0 mmHg at 25°C |
| Index of Refraction | 1.552 |
| Storage condition | -20℃ |
| Hazard Codes | Xi |
|---|---|
| Risk Phrases | R36/37/38:Irritating to eyes, respiratory system and skin . |
| Safety Phrases | 36/37/39-26 |
| WGK Germany | 3 |
| RTECS | LK8650000 |
| HS Code | 2933399090 |
|
~90% 
Ropivacaine CAS#:84057-95-4 |
| Literature: BRIDGE PHARMA, INC. Patent: WO2008/88756 A1, 2008 ; Location in patent: Page/Page column 34 ; |
|
~93% 
Ropivacaine CAS#:84057-95-4 |
| Literature: L. MOLTENI and C. DEI FRATELLI ALITTI - SOCIETA DI ESERCIZIO S.P.A. Patent: WO2006/133837 A2, 2006 ; Location in patent: Page/Page column 3-4 ; |
| HS Code | 2933399090 |
|---|---|
| Summary | 2933399090. other compounds containing an unfused pyridine ring (whether or not hydrogenated) in the structure. VAT:17.0%. Tax rebate rate:13.0%. . MFN tariff:6.5%. General tariff:20.0% |
| (S)-ROPIVACAINE HCL |
| (2S)-N-(2,6-Dimethylphenyl)-1-propylpiperidine-2-carboxamide |
| (S)-ROPIVACAINE HYDROCHLORIDE |
| (S)ROPIVACAINE HYDROCHLORIDE |
| (S)-ROPIVACAINEHYDROCHLORIDE |
| UNII-V910P86109 |
| (S)-(-)-ropivacaine |
| (-)-1-propyl-2',6'-dimethyl-2-piperidylcarboxyanilide |
| Ropivacaine |
| Ropivacaine hydrochloride Monohydrate |
| (S)ROPIVACAINE HY DROCHLORIDE |
| 2-Piperidinecarboxamide, N-(2,6-dimethylphenyl)-1-propyl-, (2S)-, hydrochloride, hydrate (1:1:1) |
| S-ROPIVACAINE HCL |
| Ropivacaine (hydrochloride Monohydrate) |
| (2S)-N-(2,6-Dimethylphenyl)-1-propyl-2-piperidinecarboxamide |
| Ropivacaine API |
| (+-)-ropivacaine hydrochloride |
| (S)-ROPIVACAINEHCL |
| (S)-(-)-1-propyl-2',6'-pipecoloxylidide |
| S-ROPIVACAINEHCL |
| ROPACARAINEHCL |
| MFCD00864425 |
| 2-Piperidinecarboxamide, N-(2,6-dimethylphenyl)-1-propyl-, (2S)- |
| RopivacaineHcl/MesylateBase |
| Ropivacainehydrochloride |
| Naropin |
| Ropivacaine hydrochloride |
| (S)-(-)-1-propyl-2',6'-pipecoloxylidine hydrochloride monohydrate |
| l-N-n-Propylpipecolic acid-2,6-xylidide |
| ROPIVACAINE MESYLATER |
| (-)-1-Propyl-2',6'-pipecoloxylidide |
| UNII-7IO5LYA57N |
| (S)-ropivacaine hydrochloride hydrate |
| (S)-N-(2,6-dimethylphenyl)-1-propyl-2-piperidinecarboxamide |
| Naropine |
| (S)-N-propylpiperidine-2-carboxylic acid 2,6-dimethylphenyl amide |
| ropivacaine hydrochloride hydrate |
| Ropivacaine HCl.H2O |
| Ropivacaine HCl |
| S-ROPIVACAINE HYDROCHLORIDE |
| (2S)-N-(2,6-Dimethylphenyl)-1-propyl-2-piperidinecarboxamide hydrochloride hydrate |
| (2S)-N-(2,6-Dimethylphenyl)-1-propylpiperidine-2-carboxamide hydrochloride hydrate |
 CAS#:132112-35-7
CAS#:132112-35-7
