684647-62-9
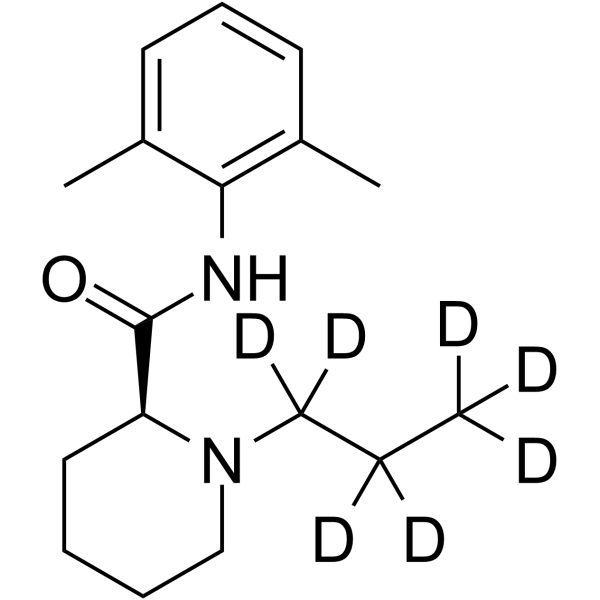
| Name | (2S)-N-(2,6-dimethylphenyl)-1-(1,1,2,2,3,3,3-heptadeuteriopropyl)piperidine-2-carboxamide |
|---|
| Description | Ropivacaine-d7 is deuterium labeled Ropivacaine. Ropivacain is a potent sodium channel blocker. Ropivacain blocks impulse conduction via reversible inhibition of sodium ion influx in nerve fibrese[1][2]. Ropivacaine is also an inhibitor of K2P (two-pore domain potassium channel) TREK-1 with an IC50 of 402.7 μM in COS-7 cell's membrane[3]. Ropivacaine is used for the research of neuropathic pain management[1]. |
|---|---|
| Related Catalog | |
| In Vitro | Stable heavy isotopes of hydrogen, carbon, and other elements have been incorporated into drug molecules, largely as tracers for quantitation during the drug development process. Deuteration has gained attention because of its potential to affect the pharmacokinetic and metabolic profiles of drugs[1]. |
| References |
| Molecular Formula | C17H19D7N2O |
|---|---|
| Molecular Weight | 281.44 |
| Exact Mass | 281.24800 |
| PSA | 35.83000 |
| LogP | 4.09380 |