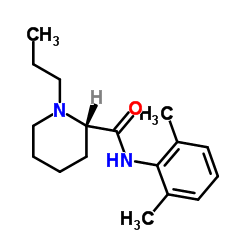
132112-35-7
| Name | (S)-ropivacaine hydrochloride hydrate |
|---|---|
| Synonyms |
(S)-ROPIVACAINE HCL
(2S)-N-(2,6-Dimethylphenyl)-1-propylpiperidine-2-carboxamide (S)-ROPIVACAINE HYDROCHLORIDE (S)ROPIVACAINE HYDROCHLORIDE (S)-ROPIVACAINEHYDROCHLORIDE UNII-V910P86109 (-)-1-propyl-2',6'-dimethyl-2-piperidylcarboxyanilide Ropivacaine Ropivacaine hydrochloride Monohydrate (S)ROPIVACAINE HY DROCHLORIDE 2-Piperidinecarboxamide, N-(2,6-dimethylphenyl)-1-propyl-, (2S)-, hydrochloride, hydrate (1:1:1) S-ROPIVACAINE HCL Ropivacaine (hydrochloride Monohydrate) (2S)-N-(2,6-Dimethylphenyl)-1-propyl-2-piperidinecarboxamide (+-)-ropivacaine hydrochloride (S)-ROPIVACAINEHCL (S)-(-)-1-propyl-2',6'-pipecoloxylidide S-ROPIVACAINEHCL Ropivacainehydrochloride Ropivacaine hydrochloride (S)-(-)-1-propyl-2',6'-pipecoloxylidine hydrochloride monohydrate l-N-n-Propylpipecolic acid-2,6-xylidide (-)-1-Propyl-2',6'-pipecoloxylidide UNII-7IO5LYA57N (S)-ropivacaine hydrochloride hydrate (S)-N-(2,6-dimethylphenyl)-1-propyl-2-piperidinecarboxamide ropivacaine hydrochloride hydrate Ropivacaine HCl.H2O Ropivacaine HCl S-ROPIVACAINE HYDROCHLORIDE (2S)-N-(2,6-Dimethylphenyl)-1-propyl-2-piperidinecarboxamide hydrochloride hydrate (2S)-N-(2,6-Dimethylphenyl)-1-propylpiperidine-2-carboxamide hydrochloride hydrate MFCD02102164 |
| Description | Ropivacaine HCl is an anaesthetic agent and blocks impulse conduction in nerve fibres through inhibiting sodium ion influx reversibly.Target: Sodium ChannelRopivacaine is a new long-acting local anesthetic, with vasoconstrictive properties. Ropivacaine given epidurally provided adequate sensory anesthesia and motor block for transurethral surgery. Addition of epinephrine did not provide any significant prolongation of the sensory or motor block, nor any influence upon the sympathetic block [1]. Ropivacaine was metabolized to 2',6'-pipecoloxylidide (PPX), 3'-hydroxyropivacaine (3'-OH Rop), and 4'-hydroxyropivacaine (4'-OH Rop) by hepatic microsomes from human and rat. Ropivacaine N-dealkylation and 3'-hydroxylation activities correlated well with the level of CYP3A4 and 1A2 in human hepatic microsomes, respectively [2]. |
|---|---|
| Related Catalog | |
| References |
| Density | 1.0±0.1 g/cm3 |
|---|---|
| Boiling Point | 410.2±45.0 °C at 760 mmHg |
| Melting Point | 267-269ºC |
| Molecular Formula | C17H29ClN2O2 |
| Molecular Weight | 328.88 |
| Flash Point | 201.9±28.7 °C |
| PSA | 41.57000 |
| LogP | 3.11 |
| Vapour Pressure | 0.0±1.0 mmHg at 25°C |
| Index of Refraction | 1.552 |
| Storage condition | Refrigerator |
| Symbol |

GHS05 |
|---|---|
| Signal Word | Danger |
| Hazard Statements | H318 |
| Precautionary Statements | P280-P305 + P351 + P338 + P310 |
| Hazard Codes | T: Toxic; |
| Risk Phrases | R26/27/28 |
| Safety Phrases | 22-36/37/39-45 |
| RIDADR | UN 2811 6.1 / PGIII |
| HS Code | 2933399090 |
|
~82% 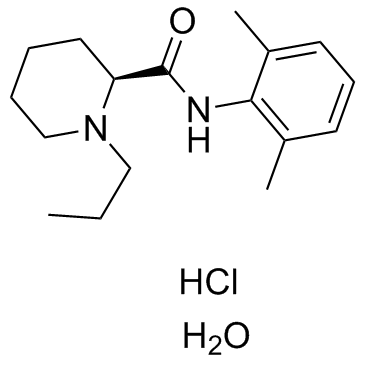
132112-35-7 |
| Literature: Navinta LLC Patent: US2006/276654 A1, 2006 ; Location in patent: Page/Page column 5 ; |
|
~% 
132112-35-7 |
| Literature: US2006/276654 A1, ; Page/Page column 5 ; |
| Precursor 2 | |
|---|---|
| DownStream 0 | |
| HS Code | 2933399090 |
|---|---|
| Summary | 2933399090. other compounds containing an unfused pyridine ring (whether or not hydrogenated) in the structure. VAT:17.0%. Tax rebate rate:13.0%. . MFN tariff:6.5%. General tariff:20.0% |