(R)-Baclofen (hydrochloride)
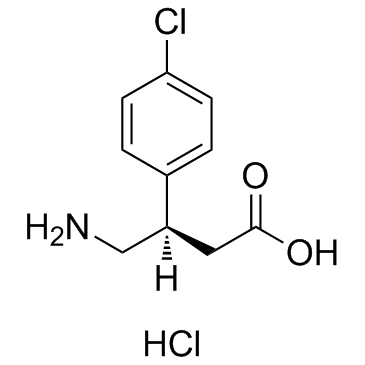
(R)-Baclofen (hydrochloride) structure
|
Common Name | (R)-Baclofen (hydrochloride) | ||
|---|---|---|---|---|
| CAS Number | 63701-55-3 | Molecular Weight | 250.12200 | |
| Density | N/A | Boiling Point | 364.3ºC at 760 mmHg | |
| Molecular Formula | C10H13Cl2NO2 | Melting Point | N/A | |
| MSDS | USA | Flash Point | 174.1ºC | |
Use of (R)-Baclofen (hydrochloride)(R)-Baclofen Hcl(STX-209 Hcl) is a derivative of gamma-aminobutyric acid (GABA) primarily used to treat spasticity and is in the early research stages for use for the treatment of alcoholism.Target: GABABaclofen (brand names Kemstro, Lioresal, Liofen, Gablofen, Beklo and Baclosan) is a derivative of gamma-aminobutyric acid (GABA). It is primarily used to treat spasticity and is in the early research stages for use for the treatment of alcoholism. It is also used by compounding pharmacies in topical pain creams as a muscle relaxant.It is an agonist for the GABAB receptors. Its beneficial effects in spasticity result from actions at spinal and supraspinal sites. Baclofen can also be used to treat hiccups, and has been shown to prevent rises in body temperature induced by the drug MDMA in rats.In addition, research has shown baclofen to be effective in the treatment of alcohol dependence and withdrawal, by inhibiting both withdrawal symptoms andcravings. |
| Name | (3R)-4-amino-3-(4-chlorophenyl)butanoic acid,hydrochloride |
|---|---|
| Synonym | More Synonyms |
| Description | (R)-Baclofen Hcl(STX-209 Hcl) is a derivative of gamma-aminobutyric acid (GABA) primarily used to treat spasticity and is in the early research stages for use for the treatment of alcoholism.Target: GABABaclofen (brand names Kemstro, Lioresal, Liofen, Gablofen, Beklo and Baclosan) is a derivative of gamma-aminobutyric acid (GABA). It is primarily used to treat spasticity and is in the early research stages for use for the treatment of alcoholism. It is also used by compounding pharmacies in topical pain creams as a muscle relaxant.It is an agonist for the GABAB receptors. Its beneficial effects in spasticity result from actions at spinal and supraspinal sites. Baclofen can also be used to treat hiccups, and has been shown to prevent rises in body temperature induced by the drug MDMA in rats.In addition, research has shown baclofen to be effective in the treatment of alcohol dependence and withdrawal, by inhibiting both withdrawal symptoms andcravings. |
|---|---|
| Related Catalog | |
| References |
| Boiling Point | 364.3ºC at 760 mmHg |
|---|---|
| Molecular Formula | C10H13Cl2NO2 |
| Molecular Weight | 250.12200 |
| Flash Point | 174.1ºC |
| Exact Mass | 249.03200 |
| PSA | 63.32000 |
| LogP | 3.35930 |
CHEMICAL IDENTIFICATION
HEALTH HAZARD DATAACUTE TOXICITY DATA
|
| Personal Protective Equipment | Eyeshields;Gloves;type N95 (US);type P1 (EN143) respirator filter |
|---|---|
| Safety Phrases | S24/25 |
| RIDADR | UN 2811 6.1/PG 3 |
| RTECS | MW5084350 |
| Precursor 8 | |
|---|---|
| DownStream 1 | |
|
Effects of phaclofen and the enantiomers of baclofen on cardiovascular responses to intrathecal administration of L- and D-baclofen in the rat.
Eur. J. Pharmacol. 196 , 267, (1991) In a previous study it was found that i.t. administration of L-baclofen decreased arterial pressure and heart rate while D-baclofen differentially increased arterial pressure. The objective of the pre... |
|
|
Comparative stereostructure-activity studies on GABAA and GABAB receptor sites and GABA uptake using rat brain membrane preparations.
J. Neurochem. 47 , 898, (1986) The affinities of a number of analogues of gamma-aminobutyric acid (GABA) for GABAA and GABAB receptor sites and GABA uptake were studied using rat brain membrane preparations. Studies on the (S)-(+)-... |
|
|
3-(p-Chlorophenyl)-4-aminobutanoic acid--resolution into enantiomers and pharmacological activity.
Pol. J. Pharmacol. Pharm. 32 , 187, (1980) Racemic 3-(p-chlorophenyl)-4-aminobutanoic acid was resolved into enantiomers and their absolute configuration determined. Pharmacological activity of hydrochlorides of the racemic acid and its enanti... |
| Arbaclofen HCl |
| d-Baclofen hydrochloride |
| MFCD00078579 |
| Arbaclofen hydrochloride |
| (R)-Baclofen (hydrochloride) |
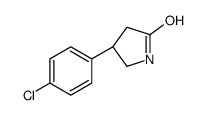 CAS#:123632-35-9
CAS#:123632-35-9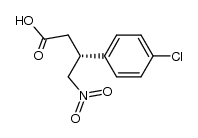 CAS#:290366-79-9
CAS#:290366-79-9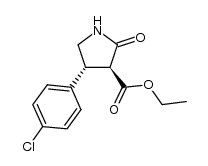 CAS#:1201795-62-1
CAS#:1201795-62-1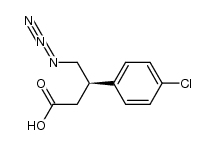 CAS#:188635-62-3
CAS#:188635-62-3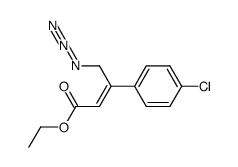 CAS#:537676-64-5
CAS#:537676-64-5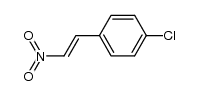 CAS#:101671-01-6
CAS#:101671-01-6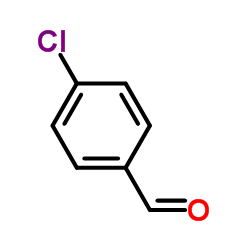 CAS#:104-88-1
CAS#:104-88-1 CAS#:1042939-73-0
CAS#:1042939-73-0 CAS#:69308-37-8
CAS#:69308-37-8