| Structure | Name/CAS No. | Articles |
|---|---|---|
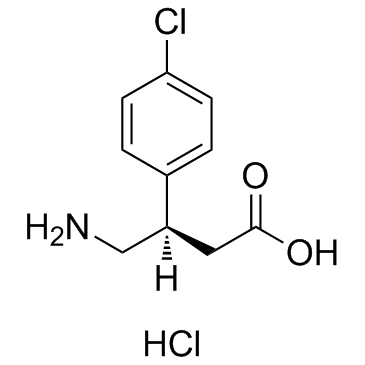 |
(R)-Baclofen (hydrochloride)
CAS:63701-55-3 |
|
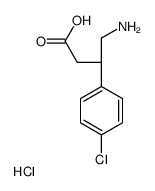 |
(S)-Baclofen hydrochloride
CAS:63701-56-4 |