Isovanillin
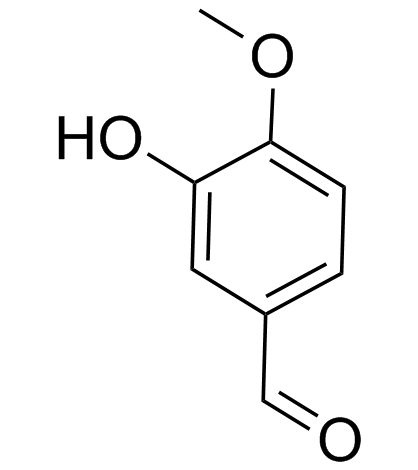
Isovanillin structure
|
Common Name | Isovanillin | ||
|---|---|---|---|---|
| CAS Number | 621-59-0 | Molecular Weight | 152.147 | |
| Density | 1.2±0.1 g/cm3 | Boiling Point | 308.1±0.0 °C at 760 mmHg | |
| Molecular Formula | C8H8O3 | Melting Point | 113-116 °C | |
| MSDS | Chinese USA | Flash Point | 119.9±15.3 °C | |
| Symbol |

GHS07 |
Signal Word | Warning | |
Use of IsovanillinIsovanillin is an aldehyde oxidase inhibitor[1]. Antispasmodic activities[2]. Antidiarrheal activities[3]. |
| Name | Isovanillin |
|---|---|
| Synonym | More Synonyms |
| Description | Isovanillin is an aldehyde oxidase inhibitor[1]. Antispasmodic activities[2]. Antidiarrheal activities[3]. |
|---|---|
| Related Catalog | |
| Target |
Aldehyde oxidase[1] |
| In Vitro | Isovanillin is not a substrate for aldehyde oxidase and therefore it is metabolized to isovanillic acid predominantly by aldehyde dehydrogenase[1]. Isovanillin is relaxant of ileum contractions induced by 5-HT (IC50=356±50μM) [2]. |
| In Vivo | Isovanillin (2 mg/kg & 5 mg/kg) and iso-acetovanillon (2 mg/kg & 5 mg/kg) both have antidiarrheal and anti-motility effect on gastrointestinal tract[3]. |
| References |
| Density | 1.2±0.1 g/cm3 |
|---|---|
| Boiling Point | 308.1±0.0 °C at 760 mmHg |
| Melting Point | 113-116 °C |
| Molecular Formula | C8H8O3 |
| Molecular Weight | 152.147 |
| Flash Point | 119.9±15.3 °C |
| Exact Mass | 152.047348 |
| PSA | 46.53000 |
| LogP | 1.10 |
| Vapour Pressure | 0.0±0.6 mmHg at 25°C |
| Index of Refraction | 1.588 |
| InChIKey | JVTZFYYHCGSXJV-UHFFFAOYSA-N |
| SMILES | COc1ccc(C=O)cc1O |
| Storage condition | -20°C Freezer, Under Inert Atmosphere |
CHEMICAL IDENTIFICATION
HEALTH HAZARD DATAACUTE TOXICITY DATA
MUTATION DATA
|
| Symbol |

GHS07 |
|---|---|
| Signal Word | Warning |
| Hazard Statements | H315-H319-H335 |
| Precautionary Statements | P261-P305 + P351 + P338 |
| Personal Protective Equipment | dust mask type N95 (US);Eyeshields;Gloves |
| Hazard Codes | Xi:Irritant |
| Risk Phrases | R36/37/38 |
| Safety Phrases | S26-S36/37 |
| RIDADR | NONH for all modes of transport |
| WGK Germany | 3 |
| RTECS | CU6540000 |
| HS Code | 29124900 |
| Precursor 10 | |
|---|---|
| DownStream 9 | |
| HS Code | 2912499000 |
|---|---|
| Summary | 2912499000. other aldehyde-ethers, aldehyde-phenols and aldehydes with other oxygen function. VAT:17.0%. Tax rebate rate:9.0%. . MFN tariff:5.5%. General tariff:30.0% |
|
Synthesis and accumulation of aromatic aldehydes in an engineered strain of Escherichia coli.
J. Am. Chem. Soc. 136(33) , 11644-54, (2014) Aromatic aldehydes are useful in numerous applications, especially as flavors, fragrances, and pharmaceutical precursors. However, microbial synthesis of aldehydes is hindered by rapid, endogenous, an... |
|
|
3D-QSAR and molecular docking studies of benzaldehyde thiosemicarbazone, benzaldehyde, benzoic acid, and their derivatives as phenoloxidase inhibitors.
Bioorg. Med. Chem. 15 , 2006-15, (2007) Phenoloxidase (PO), also known as tyrosinase, is a key enzyme in insect development, responsible for catalyzing the hydroxylation of tyrosine into o-diphenols and the oxidation of o-diphenols into o-q... |
|
|
Systemic exposure to and disposition of catechols derived from Salvia miltiorrhiza roots (Danshen) after intravenous dosing DanHong injection in human subjects, rats, and dogs.
Drug Metab. Dispos. 43(5) , 679-90, (2015) DanHong injection is a Danshen (Salvia miltiorrhiza roots)-based injectable solution for treatment of coronary artery disease and ischemic stroke. Danshen catechols are believed to be responsible for ... |
| Benzaldehyde, 3-hydroxy-4-methoxy- |
| 4-MeO-3-OHC6H3CHO |
| 3-hydroxy-4-methoxy-benzaldehyde |
| Isovanillin |
| ISOVANILIN |
| 3-Hydroxy-p-anisaldehyde |
| 4-08-00-01764 (Beilstein Handbook Reference) |
| Isovanilline |
| p-Anisaldehyde, 3-hydroxy- (8CI) |
| 3-Hydroxy-4-methoxybenzolcarbaldehyd |
| 3-Hydroxy-4-methoxybenzaldehyde |
| 4-methoxy-3-hydroxybenzaldehyde |
| EINECS 210-694-9 |
| p-Anisaldehyde, 3-hydroxy- |
| VHR CQ DO1 |
| Isovanicaline |
| MFCD00003369 |
| 5-Formylguaiacol |
| iso-vanillin |
| Isovanilllin |
 CAS#:4383-06-6
CAS#:4383-06-6 CAS#:139-85-5
CAS#:139-85-5 CAS#:74-88-4
CAS#:74-88-4 CAS#:6346-05-0
CAS#:6346-05-0 CAS#:18075-40-6
CAS#:18075-40-6 CAS#:145654-01-9
CAS#:145654-01-9 CAS#:120-57-0
CAS#:120-57-0![3-[tert-butyl(dimethyl)silyl]oxy-4-methoxybenzaldehyde Structure](https://image.chemsrc.com/caspic/199/97315-18-9.png) CAS#:97315-18-9
CAS#:97315-18-9 CAS#:5779-98-6
CAS#:5779-98-6 CAS#:111394-51-5
CAS#:111394-51-5 CAS#:110815-90-2
CAS#:110815-90-2 CAS#:105728-94-7
CAS#:105728-94-7![3-[4-(bromomethyl)phenoxy]-4-methoxybenzaldehyde structure](https://image.chemsrc.com/caspic/233/111436-61-4.png) CAS#:111436-61-4
CAS#:111436-61-4![5-[[2-(4-hydroxyphenyl)ethylamino]methyl]-2-methoxy-phenol structure](https://image.chemsrc.com/caspic/221/4579-60-6.png) CAS#:4579-60-6
CAS#:4579-60-6 CAS#:37687-57-3
CAS#:37687-57-3 CAS#:52805-46-6
CAS#:52805-46-6 CAS#:34123-66-5
CAS#:34123-66-5![4-[(2R)-2-(Chloromethyl)-3-methylbutyl]-1-methoxy-2-(3-methoxypropoxy)benzene structure](https://image.chemsrc.com/caspic/328/324763-39-5.png) CAS#:324763-39-5
CAS#:324763-39-5
