3,4-Dihydroxybenzaldehyde
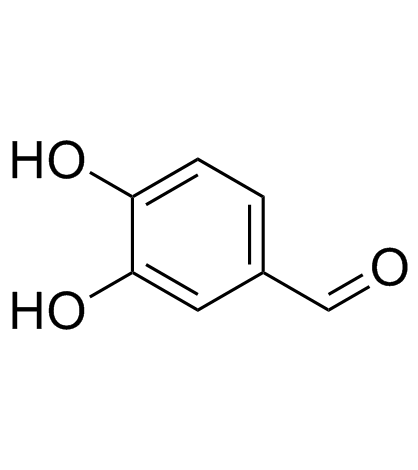
3,4-Dihydroxybenzaldehyde structure
|
Common Name | 3,4-Dihydroxybenzaldehyde | ||
|---|---|---|---|---|
| CAS Number | 139-85-5 | Molecular Weight | 138.12 | |
| Density | 1.4±0.1 g/cm3 | Boiling Point | 295.4±20.0 °C at 760 mmHg | |
| Molecular Formula | C7H6O3 | Melting Point | 150-157 °C(lit.) | |
| MSDS | Chinese USA | Flash Point | 146.7±18.3 °C | |
| Symbol |

GHS07 |
Signal Word | Warning | |
Use of 3,4-DihydroxybenzaldehydeProtocatechualdehyde (Catechaldehyde), a natural polyphenol compound isolated from the roots of radix Salviae Miltiorrhizae, is associated with a wide variety of biological activities and has been widely used in medicine as an antioxidant, anti-aging, an antibacterial and anti-inflammatory agent[1]. |
| Name | 3,4-dihydroxybenzaldehyde |
|---|---|
| Synonym | More Synonyms |
| Description | Protocatechualdehyde (Catechaldehyde), a natural polyphenol compound isolated from the roots of radix Salviae Miltiorrhizae, is associated with a wide variety of biological activities and has been widely used in medicine as an antioxidant, anti-aging, an antibacterial and anti-inflammatory agent[1]. |
|---|---|
| Related Catalog | |
| In Vitro | Protocatechualdehyde (PCA) (50, 100 μM, 24/48 hours ) treated MCF-7 cells significantly decrease cell growth by 11% and 20% in 24 hours and by 22% and 27% in 48 hours, respectively[2]. Protocatechualdehyde (50, 100 μM, 24 hours) treated MCF-7 cells are increased by 1.9-fold and 2.6-fold in the concentrations of 50 μM and 100 μM, respectively. PCA suppresses proliferation of estrogen receptor (ER)-positive (MCF-7) breast cancer cells, but not ER-negative (MDA-MB-231) breast cancer cells[2]. Protocatechualdehyde (0, 100, 200 μM, 48 hours in HCT116 and SW480 cells) affects the enzyme activity of HDAC and observed that PCA treatment resulted in inhibition of HDAC activity in dose-dependent manner[3]. Cell Proliferation Assay[2] Cell Line: Human breast cancer cell (MCF-7 and MDA-MB-231) Concentration: 0, 5, 10, 25, 50, and 100 μM Incubation Time: 24, 48 hours Result: inhibited MCF-7 cells cell growth. Apoptosis Analysis[2] Cell Line: Human breast cancer cell (MCF-7 and MDA-MB-231) Concentration: 0, 5, 10, 25, 50, and 100 μM Incubation Time: 24, 48 hours Result: increased apoptosis in MCF-7 cells. |
| References |
| Density | 1.4±0.1 g/cm3 |
|---|---|
| Boiling Point | 295.4±20.0 °C at 760 mmHg |
| Melting Point | 150-157 °C(lit.) |
| Molecular Formula | C7H6O3 |
| Molecular Weight | 138.12 |
| Flash Point | 146.7±18.3 °C |
| Exact Mass | 138.031693 |
| PSA | 57.53000 |
| LogP | 1.14 |
| Vapour Pressure | 0.0±0.6 mmHg at 25°C |
| Index of Refraction | 1.674 |
| InChIKey | IBGBGRVKPALMCQ-UHFFFAOYSA-N |
| SMILES | O=Cc1ccc(O)c(O)c1 |
| Storage condition | 2-8°C |
| Stability | Stable. Incompatible with strong bases, strong oxidizing agents. |
| Water Solubility | 50 g/L (20 ºC) |
CHEMICAL IDENTIFICATION
HEALTH HAZARD DATAACUTE TOXICITY DATA
|
| Symbol |

GHS07 |
|---|---|
| Signal Word | Warning |
| Hazard Statements | H315-H319-H335 |
| Precautionary Statements | P261-P305 + P351 + P338 |
| Personal Protective Equipment | dust mask type N95 (US);Eyeshields;Gloves |
| Hazard Codes | Xi:Irritant |
| Risk Phrases | R36/37/38 |
| Safety Phrases | S26-S36-S37/39 |
| RIDADR | NONH for all modes of transport |
| WGK Germany | 3 |
| RTECS | UL0380000 |
| HS Code | 29124900 |
| Precursor 9 | |
|---|---|
| DownStream 10 | |
| HS Code | 2912499000 |
|---|---|
| Summary | 2912499000. other aldehyde-ethers, aldehyde-phenols and aldehydes with other oxygen function. VAT:17.0%. Tax rebate rate:9.0%. . MFN tariff:5.5%. General tariff:30.0% |
|
Synthesis and accumulation of aromatic aldehydes in an engineered strain of Escherichia coli.
J. Am. Chem. Soc. 136(33) , 11644-54, (2014) Aromatic aldehydes are useful in numerous applications, especially as flavors, fragrances, and pharmaceutical precursors. However, microbial synthesis of aldehydes is hindered by rapid, endogenous, an... |
|
|
3D-QSAR and molecular docking studies of benzaldehyde thiosemicarbazone, benzaldehyde, benzoic acid, and their derivatives as phenoloxidase inhibitors.
Bioorg. Med. Chem. 15 , 2006-15, (2007) Phenoloxidase (PO), also known as tyrosinase, is a key enzyme in insect development, responsible for catalyzing the hydroxylation of tyrosine into o-diphenols and the oxidation of o-diphenols into o-q... |
|
|
Chromatographic evaluation and QSAR optimization for benzoic acid analogues against carbonic anhydrase III.
J. Enzyme Inhib. Med. Chem. 30 , 420-9, (2015) An HPLC-size exclusion method was developed as an assay method to evaluate the binding of tested compounds with carbonic anhydrase III (CAIII) enzyme. Inhibition of CAIII by a group of benzoic acid an... |
| Benzaldehyde, 3,4-dihydroxy- |
| 1,2-dihydroxy-4-formylbenzene |
| 3,4-Dihydroxy benzaldehyde |
| 4-formyl-1,2-benzenediol |
| 3,4-Dihydroxybenzyl aldehyde |
| RANCINAMYCIN IV |
| MFCD00003370 |
| UNII-4PVP2HCH4T |
| 4-formyl-1,2-dihydroxybenzene |
| EINECS 205-377-7 |
| 3,4-Dihydroxybenzaldehyde |
| Protocatechuic aldehyde |
| Protocatechualdehyde |
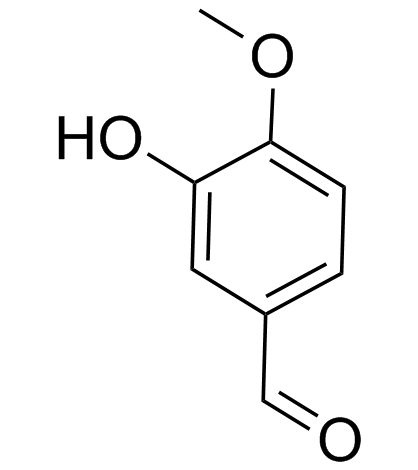 CAS#:621-59-0
CAS#:621-59-0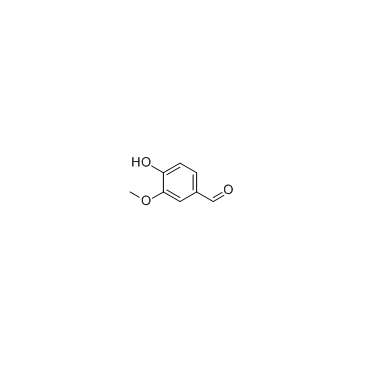 CAS#:121-33-5
CAS#:121-33-5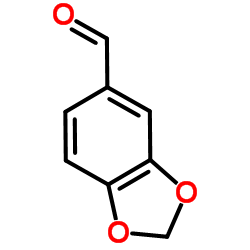 CAS#:120-57-0
CAS#:120-57-0 CAS#:859451-00-6
CAS#:859451-00-6 CAS#:869277-74-7
CAS#:869277-74-7 CAS#:869224-41-9
CAS#:869224-41-9 CAS#:775-01-9
CAS#:775-01-9 CAS#:4383-06-6
CAS#:4383-06-6 CAS#:120-80-9
CAS#:120-80-9 CAS#:10586-13-7
CAS#:10586-13-7 CAS#:10554-65-1
CAS#:10554-65-1 CAS#:34098-18-5
CAS#:34098-18-5 CAS#:54337-90-5
CAS#:54337-90-5 CAS#:3897-89-0
CAS#:3897-89-0 CAS#:3131-52-0
CAS#:3131-52-0 CAS#:4049-39-2
CAS#:4049-39-2 CAS#:50773-56-3
CAS#:50773-56-3 CAS#:5447-02-9
CAS#:5447-02-9 CAS#:3934-87-0
CAS#:3934-87-0
