Fluvoxamine maleat
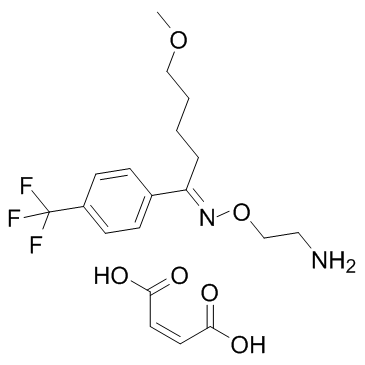
Fluvoxamine maleat structure
|
Common Name | Fluvoxamine maleat | ||
|---|---|---|---|---|
| CAS Number | 61718-82-9 | Molecular Weight | 434.407 | |
| Density | N/A | Boiling Point | 370.6ºC at 760 mmHg | |
| Molecular Formula | C19H25F3N2O6 | Melting Point | 120-121.5ºC | |
| MSDS | Chinese USA | Flash Point | 177.9ºC | |
| Symbol |

GHS07 |
Signal Word | Warning | |
Use of Fluvoxamine maleatFluvoxamine maleate is an antidepressant which functions pharmacologically as a selective serotonin reuptake inhibitor.Target: SSRIsFluvoxamine (maleate) is the maleate salt form of fluvoxamine, which is effective in inhibiting 5-HT uptake by blood platelets and brain synaptosomes. The antagonism by fluvoxamine of the reserpine-induced lowering of the pentamethylenetetrazole convulsive threshold can be regarded as due to an effect upon 5-HT uptake. In contrast to the effects of desmethylimipramine and imipramine, no stimulatory effects are found in rats when rapidly acting reserpine-like compounds are given following a dose of fluvoxamine [1]. fluvoxamine appears to improve combat-related PTSD symptoms but not depressive symptoms. The high attrition rate and lack of a placebo group limits the conclusions of our study. Controlled studies of fluvoxamine in the treatment of PTSD are warranted [2]. Fluvoxamine was less potent at decreasing ethanol self-administration when food was available concurrently versus when ethanol was available in isolation [ED50: 4.0 (2.7-5.9) and 5.1 (4.3-6.0)]. Effects on food were similar under each condition in which food was available. The results demonstrate that the potency of fluvoxamine in reducing ethanol-maintained behavior depends on whether ethanol is available in isolation or in the context of concurrently scheduled food reinforcement [3].Clinical indications: Depression; Obsessive compulsive disorder; Social phobia " FDA Approved Date: December 5, 1994Toxicity: Anorexia, Constipation, Dry mouth, Headache, Nausea, Nervousness, Skin rash, Sleep problems, Somnolence, Liver toxicity, Mania, Increase urination, Seizures, Sweating increase, Tremors, or Tourette's syndrome. |
| Name | Fluvoxamine maleate |
|---|---|
| Synonym | More Synonyms |
| Description | Fluvoxamine maleate is an antidepressant which functions pharmacologically as a selective serotonin reuptake inhibitor.Target: SSRIsFluvoxamine (maleate) is the maleate salt form of fluvoxamine, which is effective in inhibiting 5-HT uptake by blood platelets and brain synaptosomes. The antagonism by fluvoxamine of the reserpine-induced lowering of the pentamethylenetetrazole convulsive threshold can be regarded as due to an effect upon 5-HT uptake. In contrast to the effects of desmethylimipramine and imipramine, no stimulatory effects are found in rats when rapidly acting reserpine-like compounds are given following a dose of fluvoxamine [1]. fluvoxamine appears to improve combat-related PTSD symptoms but not depressive symptoms. The high attrition rate and lack of a placebo group limits the conclusions of our study. Controlled studies of fluvoxamine in the treatment of PTSD are warranted [2]. Fluvoxamine was less potent at decreasing ethanol self-administration when food was available concurrently versus when ethanol was available in isolation [ED50: 4.0 (2.7-5.9) and 5.1 (4.3-6.0)]. Effects on food were similar under each condition in which food was available. The results demonstrate that the potency of fluvoxamine in reducing ethanol-maintained behavior depends on whether ethanol is available in isolation or in the context of concurrently scheduled food reinforcement [3].Clinical indications: Depression; Obsessive compulsive disorder; Social phobia " FDA Approved Date: December 5, 1994Toxicity: Anorexia, Constipation, Dry mouth, Headache, Nausea, Nervousness, Skin rash, Sleep problems, Somnolence, Liver toxicity, Mania, Increase urination, Seizures, Sweating increase, Tremors, or Tourette's syndrome. |
|---|---|
| Related Catalog | |
| References |
| Boiling Point | 370.6ºC at 760 mmHg |
|---|---|
| Melting Point | 120-121.5ºC |
| Molecular Formula | C19H25F3N2O6 |
| Molecular Weight | 434.407 |
| Flash Point | 177.9ºC |
| Exact Mass | 434.166473 |
| PSA | 131.44000 |
| LogP | 3.61360 |
| Storage condition | 2-8°C |
| Water Solubility | H2O: soluble |
CHEMICAL IDENTIFICATION
HEALTH HAZARD DATAACUTE TOXICITY DATA
|
| Symbol |

GHS07 |
|---|---|
| Signal Word | Warning |
| Hazard Statements | H302 |
| Precautionary Statements | P301 + P312 + P330 |
| Personal Protective Equipment | dust mask type N95 (US);Eyeshields;Gloves |
| Hazard Codes | Xn:Harmful |
| Risk Phrases | R22 |
| Safety Phrases | S36 |
| RIDADR | NONH for all modes of transport |
| WGK Germany | 3 |
| RTECS | SA9230000 |
| HS Code | 2928000090 |
|
~% 
Fluvoxamine maleat CAS#:61718-82-9 |
| Literature: US6433225 B1, ; |
| Precursor 3 | |
|---|---|
| DownStream 0 | |
| HS Code | 2928000090 |
|---|---|
| Summary | 2928000090 other organic derivatives of hydrazine or of hydroxylamine VAT:17.0% Tax rebate rate:9.0% Supervision conditions:none MFN tariff:6.5% General tariff:20.0% |
|
Tacrine sinusoidal uptake and biliary excretion in sandwich-cultured primary rat hepatocytes.
J. Pharm. Pharm. Sci. 17(3) , 427-38, (2014) PURPOSE. The knowledge of hepatic disposition kinetics of tacrine, a first cholinesterase inhibitor was approved by FDA for the treatment of Alzheimer's disease (AD), would help to understand its hepa... |
|
|
5-HTTLPR rs25531A > G differentially influence paroxetine and fluvoxamine antidepressant efficacy: a randomized, controlled trial.
J. Clin. Psychopharmacol. 33(1) , 131-2, (2013)
|
|
|
Pharmacological profile of encounter-induced hyperactivity in isolation-reared mice.
Behav. Pharmacol. 26 , 681-90, (2015) We have recently found that isolation-reared mice show hyperactivity during an encounter with an intruder. However, it is not known whether encounter-induced hyperactivity may model some aspects of ps... |
| (E)-5-methoxy-1-[4-(trifluoromethyl)phenyl]-1-pentanone 2-aminoethyloxime maleate |
| MFCD00269809 |
| (2Z)-But-2-endisäure--(1E)-5-methoxy-1-[4-(trifluormethyl)phenyl]pentan-1-onO-(2-aminoethyl)oxim(1:1) |
| EINECS 228-994-3 |
| 2-[({(1E)-5-Methoxy-1-[4-(trifluoromethyl)phenyl]pentylidene}amino)oxy]ethanamine (2Z)-2-butenedioate |
| DU 23000 {Maleate} |
| faverin50 |
| Fluvoxamine Maleat |
| Fluvoxamine (maleate) |
| SME 3110 {Maleate} |
| (E)-fluvoxamine maleate |
| MK 264 {Maleate} |
| 2-[({(1E)-5-Methoxy-1-[4-(trifluoromethyl)phenyl]pentylidene}amino)oxy]ethanamine (2Z)-but-2-enedioate (1:1) |
| Fluvoxamine maleate |
| MK-264 |
| 2-{[(E)-{5-Methoxy-1-[4-(trifluoromethyl)phenyl]pentylidene}amino]oxy}ethanamine (2Z)-2-butenedioate (1:1) |
| 2-{[(E)-{5-Methoxy-1-[4-(trifluoromethyl)phenyl]pentylidene}amino]oxy}ethanamine (2Z)-but-2-enedioate (1:1) |
| (E)-5-methoxy-1-[4-(trifluoromethyl)phenyl]pentan-1-one O-2-aminoethyl oxime (Z)-but-2-enedoic acid salt |
| 1-Pentanone, 5-methoxy-1-[4-(trifluoromethyl)phenyl]-, O-(2-aminoethyl)oxime, (1E)-, (2Z)-2-butenedioate (1:1) |
| Faverin |
| Floxyfral |
| 2-[({(1E)-5-Methoxy-1-[4-(trifluoromethyl)phenyl]pentylidene}amino)oxy]ethanamine (2Z)-2-butenedioate (1:1) |
| Maveral |
| Dumirox |
| (E)-5-methoxy-1-[4-(trifluoromethyl)phenyl]pentan-1-one O-2-aminoethyl oxime maleate |
| acide (2Z)-but-2-ènedioïque - 2-[({(1E)-5-méthoxy-1-[4-(trifluorométhyl)phényl]pentylidène}amino)oxy]éthanamine (1:1) |
| Luvox |
| Fevarin |
| 2-[({(1E)-5-methoxy-1-[4-(trifluoromethyl)phenyl]pentylidene}amino)oxy]ethanamine (2Z)-but-2-enedioate |
![1-Pentanone-5-methoxy-1-[4-(trifluoromethyl)phenyl]-oxime structure](https://image.chemsrc.com/caspic/219/61747-22-6.png)