PGJ2
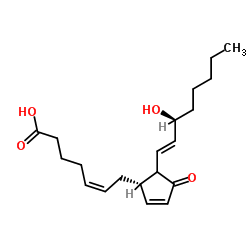
PGJ2 structure
|
Common Name | PGJ2 | ||
|---|---|---|---|---|
| CAS Number | 60203-57-8 | Molecular Weight | 334.450 | |
| Density | 1.1±0.1 g/cm3 | Boiling Point | 521.7±50.0 °C at 760 mmHg | |
| Molecular Formula | C20H30O4 | Melting Point | N/A | |
| MSDS | N/A | Flash Point | 283.4±26.6 °C | |
Use of PGJ2Prostaglandin J2 (PGJ2), an endogenous metabolite of Prostaglandin D2 (PGD2; HY-101988), is a potent PGD2 receptor (DP) agonist with Kis of 0.9 nM and 6.6 nM for hDP and hCRTH2, respectively. Prostaglandin J2 stimulates intracellular cyclic AMP production with an EC50 value of 1.2 nM. Prostaglandin J2 induces oxidative stress and neuronal apoptosis. Prostaglandin J2 induces the accumulation/aggregation of ubiquitinated (Ub) proteins. Prostaglandin J2 is highly neurotoxic and potentially contributes to many neurodegenerative conditions, including Alzheimer's (AD) and Parkinson's diseases (PD)[1][2][3][4]. |
| Name | prostaglandin J2 |
|---|---|
| Synonym | More Synonyms |
| Description | Prostaglandin J2 (PGJ2), an endogenous metabolite of Prostaglandin D2 (PGD2; HY-101988), is a potent PGD2 receptor (DP) agonist with Kis of 0.9 nM and 6.6 nM for hDP and hCRTH2, respectively. Prostaglandin J2 stimulates intracellular cyclic AMP production with an EC50 value of 1.2 nM. Prostaglandin J2 induces oxidative stress and neuronal apoptosis. Prostaglandin J2 induces the accumulation/aggregation of ubiquitinated (Ub) proteins. Prostaglandin J2 is highly neurotoxic and potentially contributes to many neurodegenerative conditions, including Alzheimer's (AD) and Parkinson's diseases (PD)[1][2][3][4]. |
|---|---|
| Related Catalog | |
| Target |
Human Endogenous Metabolite hDP:0.9 nM (Ki) hCRTH2:6.6 nM (Ki) hEP1:15.678 μM (Ki) hEP2:989 nM (Ki) hEP3:319 nM (Ki) hEP4:1065 nM (Ki) hFP:553 nM (Ki) hIP:>25 μM (Ki) hTP:6426 nM (Ki) |
| In Vivo | Prostaglandin J2 (PGJ2; 33.4 μg/注射; 单侧注射到 SNpc; 每周一次; 持续 2 或 4 周) 诱导大鼠进行性 PD 样病理,并表现出小胶质细胞和星形胶质细胞激活以及运动缺陷[4]。 Animal Model: Sixteen-week-old Sprague Dawley male rats[4] Dosage: 33.4 μg/injection Administration: Unilateral (right side) injections to the SNpc; once per week for 2 or 4 weeks Result: Induced progressive dopaminergic neuronal loss in the rat substantia nigra pars compacta (SNpc). Developed parkinsonian-like motor deficits in a progressive manner. |
| References |
| Density | 1.1±0.1 g/cm3 |
|---|---|
| Boiling Point | 521.7±50.0 °C at 760 mmHg |
| Molecular Formula | C20H30O4 |
| Molecular Weight | 334.450 |
| Flash Point | 283.4±26.6 °C |
| Exact Mass | 334.214417 |
| PSA | 74.60000 |
| LogP | 3.34 |
| Vapour Pressure | 0.0±3.1 mmHg at 25°C |
| Index of Refraction | 1.555 |
| Hazard Codes | Xn |
|---|---|
| Risk Phrases | 20/21/22-36/37/38 |
| Safety Phrases | 26-36 |
| (5Z,12ξ,13E,15S)-15-Hydroxy-11-oxoprosta-5,9,13-trien-1-oic acid |
| Prosta-5,9,13-trien-1-oic acid, 15-hydroxy-11-oxo-, (5Z,12ξ,13E,15S)- |
| (5Z,13E,15S)-15-Hydroxy-11-oxoprosta-5,9,13-trien-1-oic acid |
| Prostaglandin J2 |
| PGJ2 |
| Prosta-5,9,13-trien-1-oic acid, 15-hydroxy-11-oxo-, (5Z,13E,15S)- |
| 8-epi-15-J2t-IsoP |
| (Z)-7-[(1S,5R)-5-[(E,3S)-3-hydroxyoct-1-enyl]-4-oxocyclopent-2-en-1-yl]hept-5-enoic acid |
| prostagladin J2 |