87893-55-8
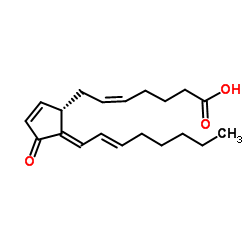
| Name | 15d-pgj2 |
|---|---|
| Synonyms |
delta12,14-Pgj 2
15-Deoxy-D-12,14-prostaglandin J2 15-Deoxy-D12,14-PGJ2 PROSTAGLANDIN J2,15-DEOXY-DELTA12,14 11-OXO-PROSTA-5Z,9,12E,14Z-TETRAEN-1-OIC 15-DEOXY-DELTA12,14-PROSTAGLANGIN J2 15-DEOXY-DELTA12,14-PGJ2 MFCD00065806 15-DEOXY-DELTA12,DELTA14-PROSTAGLANDIN J2 (5Z,12E,14E)-11-Oxo-prosta-5,9,12,14-tetraen-1-oic acid 15-Deoxy-delta12,14-prostaglandin (5Z,12E,14E)-11-Oxoprosta-5,9,12,14-tetraen-1-oic acid |
| Description | 15-Deoxy-Δ-12,14-prostaglandin J2 (15d-PGJ2) is a cyclopentenone prostaglandin and a metabolite of PGD2. 15-Deoxy-Δ-12,14-prostaglandin J2 is a selective PPARγ (EC50 of 2 µM) and a covalent PPARδ agonist. 15-Deoxy-Δ-12,14-prostaglandin J2 promotes efficient differentiation of C3H10T1/2 fibroblasts to adipocytes with an EC50 of 7 μM[1][2]. |
|---|---|
| Related Catalog | |
| Target |
PPARγ:2 μM (EC50) PPARδ Human Endogenous Metabolite |
| In Vitro | 15-Deoxy-Δ12,14-PGJ2 (15d-PGJ2) is a cyclopentenone prostaglandin that features an electrophilic, α, β-unsaturated ketone (an enone) in the cyclopentenone ring. 15-Deoxy-Δ-12,14-prostaglandin J2 is one of the cyPGs whose functions in inflammation, cell proliferation, survival, and apoptosis have been documented. 15-Deoxy-Δ-12,14-prostaglandin J2 activates PPARδ in a dose-dependent manner. 15-Deoxy-Δ-12,14-prostaglandin J2 activates PPARδ’s transcriptional activity through formation of a covalent adduct between its endocyclic enone at C9 and Cys249 in the receptor’s ligand-binding domain[1]. |
| References |
| Density | 1.1±0.1 g/cm3 |
|---|---|
| Boiling Point | 495.8±34.0 °C at 760 mmHg |
| Molecular Formula | C20H28O3 |
| Molecular Weight | 316.435 |
| Flash Point | 267.8±22.2 °C |
| Exact Mass | 316.203857 |
| PSA | 54.37000 |
| LogP | 5.10 |
| Vapour Pressure | 0.0±2.7 mmHg at 25°C |
| Index of Refraction | 1.558 |
| Symbol |


GHS02, GHS07 |
|---|---|
| Signal Word | Danger |
| Hazard Statements | H225-H319-H336 |
| Supplemental HS | Repeated exposure may cause skin dryness or cracking. |
| Precautionary Statements | P210-P280-P304 + P340 + P312-P305 + P351 + P338-P337 + P313-P403 + P235 |
| Personal Protective Equipment | Eyeshields;Faceshields;full-face respirator (US);Gloves;multi-purpose combination respirator cartridge (US) |
| Hazard Codes | F: Flammable;Xi: Irritant; |
| Risk Phrases | R11 |
| Safety Phrases | 16-26-29-33-36/37/39 |
| RIDADR | UN 1231 |
| Precursor 9 | |
|---|---|
| DownStream 0 | |
![methyl [(1S,4S)-4-[(tert-butyldimethylsilyl)oxy]-2-cyclopenten-1-yl]acetate structure](https://image.chemsrc.com/caspic/201/660430-04-6.png)
![dimethyl (1R,4S)-4-[(tert-butyldimethylsilyl)oxy]-2-cyclopenten-1-yl malonate structure](https://image.chemsrc.com/caspic/262/426226-00-8.png)
![[(1S,4S)-4-[(tert-butyldimethylsilyl)oxy]-2-cyclopenten-1-yl]ethanal structure](https://image.chemsrc.com/caspic/150/676235-98-6.png)
![(4R,2'Z)-4-[7'-(4-methoxybenzyloxy)-2'-heptenyl]-2-cyclopenten-1-one structure](https://image.chemsrc.com/caspic/453/676236-01-4.png)
![(1S,4R,2'Z)-4-[7'-(4-methoxybenzyloxy)-2'-heptenyl]-2-cyclopenten-1-ol structure](https://image.chemsrc.com/caspic/394/869801-10-5.png)
