2-Oxobutyric acid
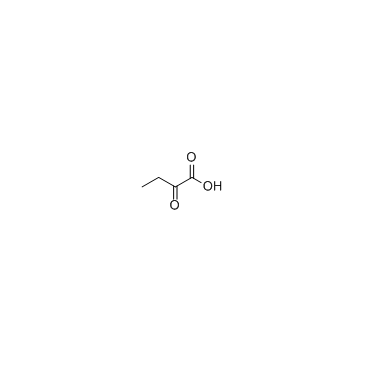
2-Oxobutyric acid structure
|
Common Name | 2-Oxobutyric acid | ||
|---|---|---|---|---|
| CAS Number | 600-18-0 | Molecular Weight | 102.089 | |
| Density | 1.2±0.1 g/cm3 | Boiling Point | 177.8±0.0 °C at 760 mmHg | |
| Molecular Formula | C4H6O3 | Melting Point | 30-34 °C(lit.) | |
| MSDS | Chinese USA | Flash Point | 65.0±15.2 °C | |
| Symbol |

GHS07 |
Signal Word | Warning | |
Use of 2-Oxobutyric acid2-Oxobutanoic acid is a product in the enzymatic cleavage of cystathionine. |
| Name | 2-oxobutanoic acid |
|---|---|
| Synonym | More Synonyms |
| Description | 2-Oxobutanoic acid is a product in the enzymatic cleavage of cystathionine. |
|---|---|
| Related Catalog | |
| Target |
Human Endogenous Metabolite |
| In Vitro | 2-Oxobutanoic acid (alpha-Ketobutyric acid) is a product in the enzymatic cleavage of cystathionine. 2-Oxobutanoic acid is a substance that is involved in the metabolism of many amino acids as well as propanoate metabolism and C-5 branched dibasic acid metabolism. 2-Oxobutanoic acid is also one of the degradation products of threonine. It can be converted into propionyl-CoA (and subsequently methylmalonyl CoA, which can be converted into succinyl CoA, a citric acid cycle intermediate), and thus enter the citric acid cycle[1]. |
| References |
| Density | 1.2±0.1 g/cm3 |
|---|---|
| Boiling Point | 177.8±0.0 °C at 760 mmHg |
| Melting Point | 30-34 °C(lit.) |
| Molecular Formula | C4H6O3 |
| Molecular Weight | 102.089 |
| Flash Point | 65.0±15.2 °C |
| Exact Mass | 102.031693 |
| PSA | 54.37000 |
| LogP | -0.71 |
| Vapour Pressure | 0.5±0.6 mmHg at 25°C |
| Index of Refraction | 1.427 |
| Storage condition | −20°C |
| Symbol |

GHS07 |
|---|---|
| Signal Word | Warning |
| Hazard Statements | H315-H319-H335 |
| Precautionary Statements | P305 + P351 + P338 |
| Personal Protective Equipment | Eyeshields;full-face respirator (US);Gloves;multi-purpose combination respirator cartridge (US);type ABEK (EN14387) respirator filter |
| Hazard Codes | Xi:Irritant; |
| Risk Phrases | R36/37/38 |
| Safety Phrases | S26-S36/37/39 |
| RIDADR | 1759 |
| WGK Germany | 3 |
| Packaging Group | III |
| Hazard Class | 8 |
| HS Code | 2918300090 |
| Precursor 8 | |
|---|---|
| DownStream 10 | |
| HS Code | 2918300090 |
|---|---|
| Summary | 2918300090 other carboxylic acids with aldehyde or ketone function but without other oxygen function, their anhydrides, halides, peroxides, peroxyacids and their derivatives。Supervision conditions:None。VAT:17.0%。Tax rebate rate:9.0%。MFN tariff:6.5%。General tariff:30.0% |
|
Mosquito odorant receptor for DEET and methyl jasmonate.
Proc. Natl. Acad. Sci. U. S. A. 111(46) , 16592-7, (2014) Insect repellents are important prophylactic tools for travelers and populations living in endemic areas of malaria, dengue, encephalitis, and other vector-borne diseases. DEET (N,N-diethyl-3-methylbe... |
|
|
Kinetic resolution of 2-hydroxybutanoate racemic mixtures by NAD-independent L-lactate dehydrogenase.
Bioresour. Technol. 102(7) , 4595-9, (2011) Optically active D-2-hydroxybutanoate is an important building block intermediate for medicines and biodegradable poly(2-hydroxybutanoate). Kinetic resolution of racemic 2-hydroxybutanoate may be a gr... |
|
|
Transaminase-catalyzed asymmetric synthesis of L-2-aminobutyric acid from achiral reactants.
Biotechnol. Lett. 31 , 1595-1599, (2009) Asymmetric synthesis of an unnatural amino acid was demonstrated by omega-transaminase from Vibrio fluvialis JS17. L-2-Aminobutyric acid was synthesized from 2-oxobutyric acid and benzylamine with an ... |
| Butanoic acid, 2-oxo- |
| EINECS 209-986-9 |
| α-oxobutyric acid |
| 2-oxo-Butyric acid |
| a-keto-n-butyric acid |
| MFCD00004164 |
| methyl-Pyruvic acid |
| propionyl- Formic acid |
| a-Oxobutyric acid |
| 2-Oxobutyric acid |
| a-oxo-n-butyric acid |
| 2-Oxobutanoic acid |
| a-ketobutyric acid |
 CAS#:20016-85-7
CAS#:20016-85-7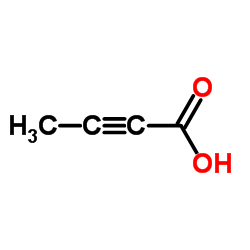 CAS#:590-93-2
CAS#:590-93-2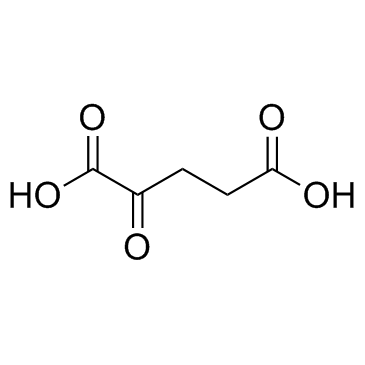 CAS#:328-50-7
CAS#:328-50-7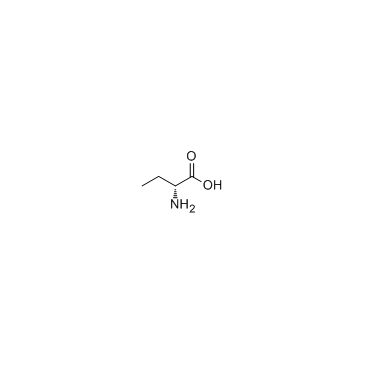 CAS#:2623-91-8
CAS#:2623-91-8 CAS#:22059-21-8
CAS#:22059-21-8 CAS#:88126-31-2
CAS#:88126-31-2 CAS#:600-15-7
CAS#:600-15-7 CAS#:642-93-3
CAS#:642-93-3 CAS#:3952-66-7
CAS#:3952-66-7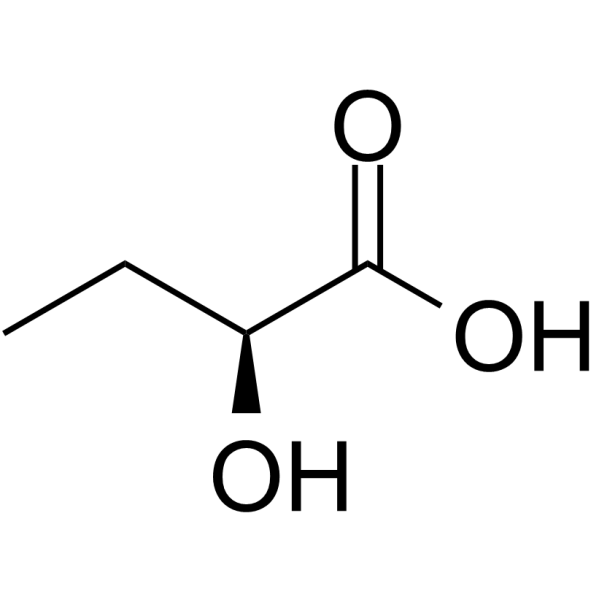 CAS#:3347-90-8
CAS#:3347-90-8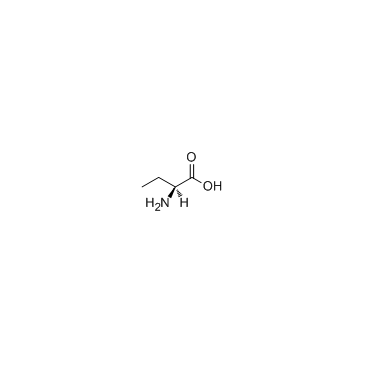 CAS#:1492-24-6
CAS#:1492-24-6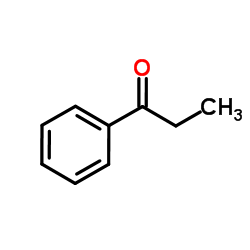 CAS#:93-55-0
CAS#:93-55-0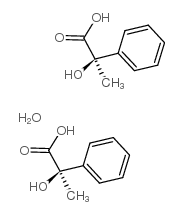 CAS#:515-30-0
CAS#:515-30-0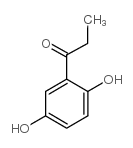 CAS#:938-46-5
CAS#:938-46-5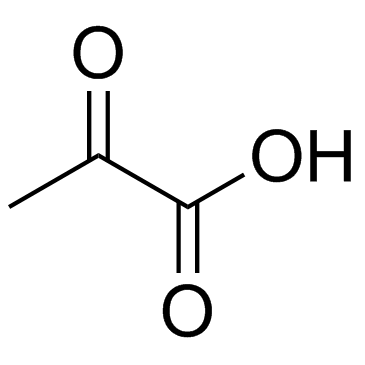 CAS#:127-17-3
CAS#:127-17-3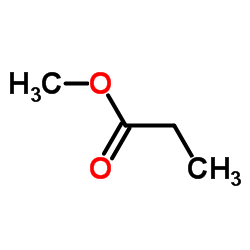 CAS#:554-12-1
CAS#:554-12-1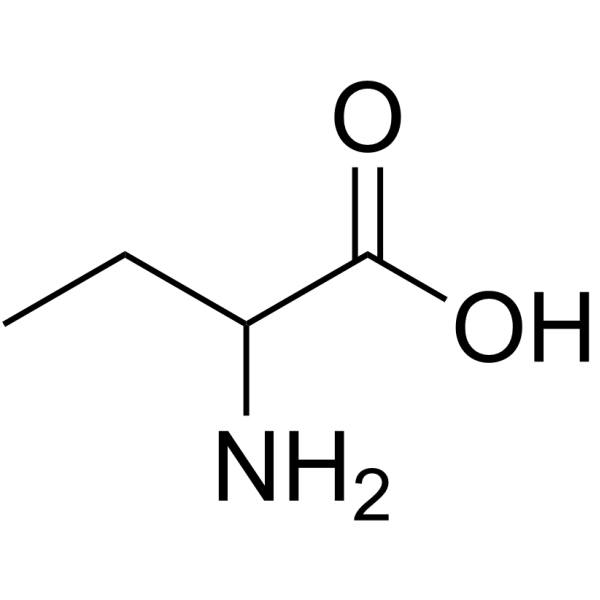 CAS#:2835-81-6
CAS#:2835-81-6
