Propiverine hydrochloride
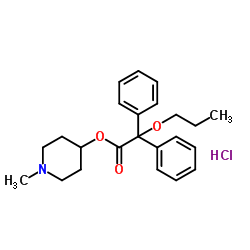
Propiverine hydrochloride structure
|
Common Name | Propiverine hydrochloride | ||
|---|---|---|---|---|
| CAS Number | 54556-98-8 | Molecular Weight | 403.942 | |
| Density | N/A | Boiling Point | 494.7ºC at 760 mmHg | |
| Molecular Formula | C23H30ClNO3 | Melting Point | 213-216ºC | |
| MSDS | Chinese USA | Flash Point | 253ºC | |
| Symbol |


GHS05, GHS07 |
Signal Word | Danger | |
Use of Propiverine hydrochloridePropiverine hydrochloride is a bladder spasmolytic with calcium antagonistic and anticholinergic properties. Propiverine hydrochloride can be used for the research of overactive blaqdder and urinary incontinence[1][2]. |
| Name | Propiverine Hydrochloride |
|---|---|
| Synonym | More Synonyms |
| Description | Propiverine hydrochloride is a bladder spasmolytic with calcium antagonistic and anticholinergic properties. Propiverine hydrochloride can be used for the research of overactive blaqdder and urinary incontinence[1][2]. |
|---|---|
| Related Catalog | |
| In Vitro | Propiverine (10-3000 nM) inhibits the specific binding of [3H]NMS, with Kis of 339, 193 and 497 nM in the bladder, submaxillary gland and heart of mice respectively[2]. |
| In Vivo | Propiverine (0.5 mg/day; p.o. once daily for 2 weeks) significantly increases UBP and LPP during passive intravesical pressure elevation, and also increases plasma norepinephrine and epinephrine levelsin rats[1]. Propiverine (0.01-1 mg/kg; i.v.) decreases the UBP and totally suppresses the sneeze reflex at the dose of 1 mg/kg in vivo[1]. Animal Model: Female adult Sprague-Dawley rats (250-270 g)[1] Dosage: 5 mg dissolved in distilled water (0.5 mL) Administration: P.o. once daily for 2 weeks Result: Increased urethral baseline pressure (UBP) and leak-point pressure (LPP) significantly. Increased plasma epinephrine and norepinephrine levels. No significant changes were observed in body weight. |
| References |
| Boiling Point | 494.7ºC at 760 mmHg |
|---|---|
| Melting Point | 213-216ºC |
| Molecular Formula | C23H30ClNO3 |
| Molecular Weight | 403.942 |
| Flash Point | 253ºC |
| Exact Mass | 403.191437 |
| PSA | 38.77000 |
| LogP | 4.73410 |
| InChIKey | KFUJMHHNLGCTIJ-UHFFFAOYSA-N |
| SMILES | CCCOC(C(=O)OC1CCN(C)CC1)(c1ccccc1)c1ccccc1.Cl |
| Storage condition | -20°C Freezer |
CHEMICAL IDENTIFICATION
HEALTH HAZARD DATAACUTE TOXICITY DATA
|
| Symbol |


GHS05, GHS07 |
|---|---|
| Signal Word | Danger |
| Hazard Statements | H315-H318-H335 |
| Precautionary Statements | P261-P280-P305 + P351 + P338 |
| Hazard Codes | Xi |
| Risk Phrases | 37/38-41 |
| Safety Phrases | 26-39 |
| RIDADR | NONH for all modes of transport |
| RTECS | AH3297000 |
| HS Code | 2933399090 |
|
~% 
Propiverine hyd... CAS#:54556-98-8 |
| Literature: WO2011/114195 A1, ; Page/Page column 6-7 ; |
| Precursor 1 | |
|---|---|
| DownStream 1 | |
| HS Code | 2933399090 |
|---|---|
| Summary | 2933399090. other compounds containing an unfused pyridine ring (whether or not hydrogenated) in the structure. VAT:17.0%. Tax rebate rate:13.0%. . MFN tariff:6.5%. General tariff:20.0% |
|
Excitatory effect of propiverine hydrochloride on urethral activity in rats.
Int. J. Urol. 19(6) , 575-82, (2012) To investigate the effects of the antimuscarinic agent, propiverine, on the bladder and urethra in rats.A total of 54 female rats were given propiverine, imidafenacin (an antimuscarinic agent), or dis... |
|
|
[Additional effect of propiverine for naftopidil-resistant nocturia in the patient with benign prostate hypertrophy].
Hinyokika Kiyo. 57(2) , 71-6, (2011) The efficacy and safety of additional administration of propiverine were prospectively studied for naftopidil-resistant nocturia in patients with benign prostatic hypertrophy (BPH). Patients of 50 yea... |
|
|
An overview on mixed action drugs for the treatment of overactive bladder and detrusor overactivity.
Urol. Int. 89(3) , 259-69, (2012) To provide an overview on the efficacy, tolerability, safety and health-related quality of life (HRQoL) of drugs with a mixed action used in the treatment of overactive bladder (OAB).MEDLINE database ... |
| Mictonetten |
| Mictonorm |
| Propiverine hydrochloride |
| Propiverine Hydrochlorride |
| 1-Methyl-4-piperidinyl diphenyl(propoxy)acetate hydrochloride (1:1) |
| Propiverine HCl |
| UNII-DC4GZD10H3 |
| Benzeneacetic acid, α-phenyl-α-propoxy-, 1-methyl-4-piperidinyl ester, hydrochloride (1:1) |
| 1-Methylpiperidin-4-yl diphenyl(propoxy)acetate hydrochloride (1:1) |

 CAS#:60569-19-9
CAS#:60569-19-9