(L)-Canavanine
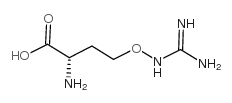
(L)-Canavanine structure
|
Common Name | (L)-Canavanine | ||
|---|---|---|---|---|
| CAS Number | 543-38-4 | Molecular Weight | 176.17 | |
| Density | 1.61g/cm3 | Boiling Point | 431.2ºC at 760mmHg | |
| Molecular Formula | C5H12N4O3 | Melting Point | 184ºC | |
| MSDS | Chinese USA | Flash Point | 214.6ºC | |
| Symbol |

GHS07 |
Signal Word | Warning | |
Use of (L)-CanavanineL-Canavanine is aamino acids and their derivatives. |
| Name | L-canavanine |
|---|---|
| Synonym | More Synonyms |
| Description | L-Canavanine is aamino acids and their derivatives. |
|---|---|
| Related Catalog |
| Density | 1.61g/cm3 |
|---|---|
| Boiling Point | 431.2ºC at 760mmHg |
| Melting Point | 184ºC |
| Molecular Formula | C5H12N4O3 |
| Molecular Weight | 176.17 |
| Flash Point | 214.6ºC |
| Exact Mass | 176.09100 |
| PSA | 134.45000 |
| LogP | 0.09430 |
| Index of Refraction | 1.602 |
CHEMICAL IDENTIFICATION
HEALTH HAZARD DATAACUTE TOXICITY DATA
MUTATION DATA
|
| Symbol |

GHS07 |
|---|---|
| Signal Word | Warning |
| Hazard Statements | H302-H312-H332 |
| Precautionary Statements | P280 |
| Personal Protective Equipment | dust mask type N95 (US);Eyeshields;Gloves |
| Hazard Codes | Xn |
| Risk Phrases | 20/21/22 |
| RIDADR | NONH for all modes of transport |
| RTECS | ES7002000 |
| HS Code | 2925290090 |
|
~% 
(L)-Canavanine CAS#:543-38-4 |
| Literature: Kitagawa; Takani Journal of Biochemistry (Tokyo, Japan), 1936 , vol. 23, p. 181,182 |
| HS Code | 2925290090 |
|---|---|
| Summary | 2925290090 other imines and their derivatives; salts thereof。Supervision conditions:None。VAT:17.0%。Tax rebate rate:9.0%。MFN tariff:6.5%。General tariff:30.0% |
|
Rad23 interaction with the proteasome is regulated by phosphorylation of its ubiquitin-like (UbL) domain.
J. Mol. Biol. 426(24) , 4049-60, (2014) Rad23 was identified as a DNA repair protein, although a role in protein degradation has been described. The protein degradation function of Rad23 contributes to cell cycle progression, stress respons... |
|
|
Shrimp waste extract and astaxanthin: rat alveolar macrophage, oxidative stress and inflammation.
J. Food Sci. 77(7) , H141-6, (2012) Astaxanthin is a carotenoid known to have antioxidant and antiinflammatory properties. This study examined if shrimp astaxanthin modulates the production of superoxide (O(-)(2)), nitric oxide (NO), an... |
|
|
Interactions between phytochemical components of Sutherlandia frutescens and the antiretroviral, atazanavir in vitro: implications for absorption and metabolism.
J. Pharm. Pharm. Sci. 15(2) , 221-33, (2012) African traditional medicinal plants, such as Sutherlandia frutescens have the potential to interact pharmacokinetically with the protease inhibitor class of antiretrovirals, thereby impacting on thei... |
| L-Canavanine |
| L-α-AMINO-γ-[GUANIDINOOXY]-N-BUTYRIC ACID |
![4-nitro-N-[[(2Z)-2-[(4-nitrophenyl)hydrazinylidene]ethylidene]amino]aniline structure](https://image.chemsrc.com/caspic/081/3078-13-5.png) CAS#:3078-13-5
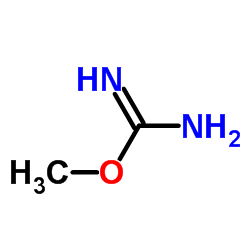
CAS#:3078-13-5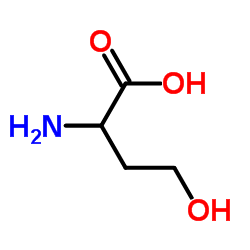 CAS#:1927-25-9
CAS#:1927-25-9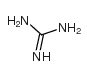 CAS#:113-00-8
CAS#:113-00-8 CAS#:7664-41-7
CAS#:7664-41-7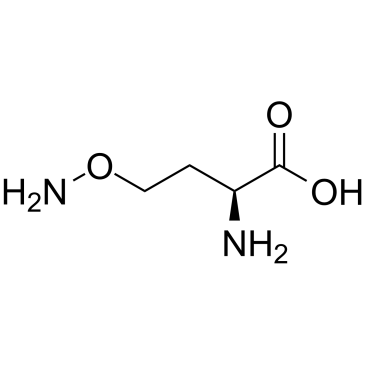 CAS#:496-93-5
CAS#:496-93-5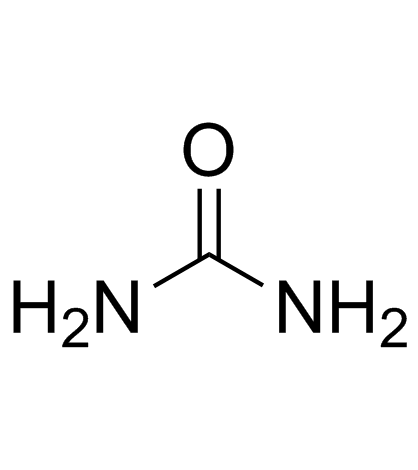 CAS#:57-13-6
CAS#:57-13-6