Marein
Modify Date: 2025-08-22 10:13:31
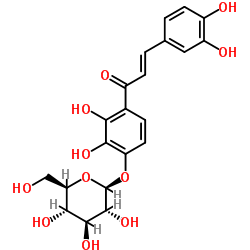
Marein structure
|
Common Name | Marein | ||
|---|---|---|---|---|
| CAS Number | 535-96-6 | Molecular Weight | 450.393 | |
| Density | 1.7±0.1 g/cm3 | Boiling Point | 835.4±65.0 °C at 760 mmHg | |
| Molecular Formula | C21H22O11 | Melting Point | 197-202ºC | |
| MSDS | N/A | Flash Point | 293.5±27.8 °C | |
Use of MareinMarein has the neuroprotective effect due to a reduction of damage to mitochondria function and activation of the AMPK signal pathway. Marein improves insulin resistance induced by high glucose in HepG2 cells through CaMKK/AMPK/GLUT1 to promote glucose uptake, through IRS/Akt/GSK-3β to increase glycogen synthesis, and through Akt/FoxO1 to decrease gluconeogenesis. Marein is a HDAC inhibitor with an IC50 of 100 µM. Marein has beneficial antioxidative, antihypertensive, antihyperlipidemic and antidiabetic effects[1][2][3]. |
| Name | marein |
|---|---|
| Synonym | More Synonyms |
| Description | Marein has the neuroprotective effect due to a reduction of damage to mitochondria function and activation of the AMPK signal pathway. Marein improves insulin resistance induced by high glucose in HepG2 cells through CaMKK/AMPK/GLUT1 to promote glucose uptake, through IRS/Akt/GSK-3β to increase glycogen synthesis, and through Akt/FoxO1 to decrease gluconeogenesis. Marein is a HDAC inhibitor with an IC50 of 100 µM. Marein has beneficial antioxidative, antihypertensive, antihyperlipidemic and antidiabetic effects[1][2][3]. |
|---|---|
| Related Catalog | |
| Target |
IC50: 100 µM (HDAC)[3] |
| References |
| Density | 1.7±0.1 g/cm3 |
|---|---|
| Boiling Point | 835.4±65.0 °C at 760 mmHg |
| Melting Point | 197-202ºC |
| Molecular Formula | C21H22O11 |
| Molecular Weight | 450.393 |
| Flash Point | 293.5±27.8 °C |
| Exact Mass | 450.116211 |
| PSA | 197.37000 |
| LogP | -0.18 |
| Vapour Pressure | 0.0±3.2 mmHg at 25°C |
| Index of Refraction | 1.751 |
| InChIKey | XGEYXJDOVMEJNG-HTFDPZBKSA-N |
| SMILES | O=C(C=Cc1ccc(O)c(O)c1)c1ccc(OC2OC(CO)C(O)C(O)C2O)c(O)c1O |
| Hazard Codes | Xi |
|---|
| 4-[(2E)-3-(3,4-Dihydroxyphenyl)-2-propenoyl]-2,3-dihydroxyphenyl β-D-glucopyranoside |
| β-D-Glucopyranoside de 4-[(2E)-3-(3,4-dihydroxyphényl)-2-propènan-1-oyl]-2,3-dihydroxyphényle |
| Okanin-4'-O-glucoside |
| (2E)-3-(3,4-Dihydroxyphenyl)-1-(2,3-dihydroxy-4-{[(2S,3R,4S,5S,6R)-3,4,5-trihydroxy-6-(hydroxymethyl)tetrahydro-2H-pyran-2-yl]oxy}phenyl)-2-propen-1-one |
| (E)-3-(3,4-Dihydroxyphenyl)-1-(4-(β-D-glucopyranosyloxy)-2,3-dihydroxyphenyl)-2-propen-1-one |
| 2-Propen-1-one, 3-(3,4-dihydroxyphenyl)-1-(4-(β-D-glucopyranosyloxy)-2,3-dihydroxyphenyl)-, (E)- |
| (2E)-3-(3,4-Dihydroxyphényl)-1-(2,3-dihydroxy-4-{[(2S,3R,4S,5S,6R)-3,4,5-trihydroxy-6-(hydroxyméthyl)tétrahydro-2H-pyran-2-yl]oxy}phényl)-2-propén-1-one |
| 4-[(2E)-3-(3,4-Dihydroxyphenyl)prop-2-enoyl]-2,3-dihydroxyphenyl β-D-glucopyranoside |
| (2E)-3-(3,4-Dihydroxyphenyl)-1-(2,3-dihydroxy-4-{[(2S,3R,4S,5S,6R)-3,4,5-trihydroxy-6-(hydroxymethyl)tetrahydro-2H-pyran-2-yl]oxy}phenyl)-2-propen-1-on |
| 2-Propen-1-one, 3-(3,4-dihydroxyphenyl)-1-[4-(β-D-glucopyranosyloxy)-2,3-dihydroxyphenyl]-, (2E)- |
| 4-[(2E)-3-(3,4-Dihydroxyphenyl)prop-2-enoyl]-2,3-dihydroxyphenyl b-D-glucopyranoside |
| Okanin-4'-glucoside |
| 4-[(2E)-3-(3,4-Dihydroxyphenyl)-2-propenoyl]-2,3-dihydroxyphenyl-β-D-glucopyranoside |
| Marein |
| 2',3,3',4,4'-pentahydroxy-4'-glucosylchalcone |