Dehydroepiandrosterone
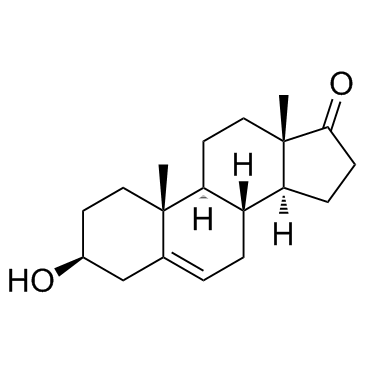
Dehydroepiandrosterone structure
|
Common Name | Dehydroepiandrosterone | ||
|---|---|---|---|---|
| CAS Number | 53-43-0 | Molecular Weight | 288.424 | |
| Density | 1.1±0.1 g/cm3 | Boiling Point | 426.7±45.0 °C at 760 mmHg | |
| Molecular Formula | C19H28O2 | Melting Point | 146-151ºC | |
| MSDS | Chinese USA | Flash Point | 182.1±21.3 °C | |
| Symbol |



GHS02, GHS06, GHS08 |
Signal Word | Danger | |
Use of DehydroepiandrosteroneDHEA is one of the most abundant steroid hormones. DHEA mediates its action via multiple signaling pathways involving specific membrane receptors and via transformation into androgen and estrogen derivatives (e.g., androgens, estrogens, 7α and 7β DHEA, and 7α and 7β epiandrosterone derivatives) acting through their specific receptors. |
| Name | dehydroepiandrosterone |
|---|---|
| Synonym | More Synonyms |
| Description | DHEA is one of the most abundant steroid hormones. DHEA mediates its action via multiple signaling pathways involving specific membrane receptors and via transformation into androgen and estrogen derivatives (e.g., androgens, estrogens, 7α and 7β DHEA, and 7α and 7β epiandrosterone derivatives) acting through their specific receptors. |
|---|---|
| Related Catalog | |
| Target |
Human Endogenous Metabolite |
| In Vitro | DHEA is an effective antiapoptotic factor, reversing the serum deprivation-induced apoptosis in prostate cancer cells (DU145 and LNCaP cell lines) as well as in colon cancer cells (Caco2 cell line). DHEA significantly reduces serum deprivation-induced apoptosis in all 3 cancer cell types, quantitated with the APOPercentage assay (apoptosis is reduced from 0.587±0.053 to 0.142±0.0016 or 0.059±0.002 after treatment for 12 hours with DHEA or NGF, respectively; n=3, P<0.01), and by flow cytometry analysis (FACS) for DU145 cells. The antiapoptotic effect of DHEA is dose dependent with an EC50 at nanomolar concentrations (EC50: 11.2±3.6 nM and 12.4±2.2 nM in DU145 and Caco2 cells, respectively)[1]. DHEA is the principal sex steroid precursor in humans and can be converted directly to androgens. DHEA (≥1 μM) causes a dose-dependent inhibition of Chub-S7 proliferation, as assessed by thymidine incorporation assays. DHEA treatment inhibits expression of the key glucocorticoid-regulating genes H6PDH (≥100 nM) and HSD11B1 (≥1 μM) in differentiating preadipocytes in a dose-dependent manner. In keeping with this finding, DHEA treatment (≥1 μM) results in a marked reduction in 11β-HSD1 oxoreductase activity (≥1 μM) and a concurrent increase in dehydrogenase activity at the highest DHEA dose used (25 μM DHEA) in differentiated adipocytes[2]. |
| In Vivo | DHEA in the diet (0.45 % w/w) of male B6 mice (groups of five mice) treated for 8 weeks led to significant decreases in body temperature compared with mice fed the control AIN-76A diet. A similar comparison indicated that control and pair-fed mice are also significantly different. Animals fed DHEA have significantly lower temperatures than mice fed the control diet 26/29 times tested; mice pair fed to those on the DHEA diet are less affected, with 8/29 values significantly lower than in mice fed AIN-76A ad libitum. The temperatures of mice fed DHEA or pair fed to DHEA are significantly different 21/29 times tested. Body weights are significantly greater in mice fed the control diet than in mice fed DHEA or pair fed to DHEA. Food intake (grams per day) from cages are averaged for each week (n=7), except for Week 9 (n=3). The amount of food intake is significantly decreased in mice fed DHEA. By design, mice pair fed to DHEA ate about the same amount. Thus, it appears that DHEA reduces body temperature by food restriction and by a separate mechanism[3]. |
| Kinase Assay | Chub-S7 cells are incubated in DMEM containing cold DHEA (20 nM) and tritiated DHEA (0.2 μCi/well) for 48 h. Following incubation, steroids are extracted using dichloromethane separated by thin-layer chromatography using n-hexane/1-hexanol (75:25) as the mobile phase system. Metabolites are identified by comigration with unlabeled reference steroids that are visualized by exposure to Lieberman-Burchard reagent (ethanol-acetic anhydride-sulfuric acid). Steroid conversion is quantified using a LabLogic AR-200 scanner. Protein concentration is measured using a colorimetric 96-well plate assay and used to normalize conversion. Activity is expressed as percent conversion[2]. |
| Cell Assay | Chub-S7 preadipocytes and human primary preadipocytes are seeded into a 24-well plate at densities 1×105 and 2.5×105 respectively. Following overnight culture, medium is supplemented with DHEA, androstenediol, or DHEAS (0-100 μM). Following 24-, 48-, or 72 h incubation, cell proliferation is assessed by incubation with radiolabeled thymidine (0.2 μCi/well) for the final 6 h of culture. Proteins are precipitated with TCA, and cells are scraped in NaOH. The respective content of radiolabeled nuclear material in the resulting lysates is analyzed by scintillation counting. Data are expressed as percentage of control[2]. |
| Animal Admin | Mice[3] Mice are fed Purina Lab Chow until the start of experiments (Day 0). Groups of five mice are then fed pelleted AIN-76A diet containing either no additive or DHEA (0.45% w/w) between 0900 and 1000 hr. Diets are stored at 4°C for no longer than six months to maintain optimal activity. Mice are given the diets ad libitum, except for mice that are pair fed to mice treated with DHEA. The amounts of AIN-76A diet the pair-fed mice received are determined by the weight of food consumed by the DHEA-fed mice on a daily basis. Body weights (grams) are measured at different time points starting at Day 1 and ending at Day 59. Daily food intakes (grams per day) are determined by weighing the food consumed per cage of five mice. The mean±SEM values are calculated for weeks 1 to 8 (n=7); week 9 had only 3 days. |
| References |
| Density | 1.1±0.1 g/cm3 |
|---|---|
| Boiling Point | 426.7±45.0 °C at 760 mmHg |
| Melting Point | 146-151ºC |
| Molecular Formula | C19H28O2 |
| Molecular Weight | 288.424 |
| Flash Point | 182.1±21.3 °C |
| Exact Mass | 288.208923 |
| PSA | 37.30000 |
| LogP | 3.42 |
| Vapour Pressure | 0.0±2.3 mmHg at 25°C |
| Index of Refraction | 1.560 |
| InChIKey | FMGSKLZLMKYGDP-USOAJAOKSA-N |
| SMILES | CC12CCC3C(CC=C4CC(O)CCC43C)C1CCC2=O |
CHEMICAL IDENTIFICATION
HEALTH HAZARD DATAACUTE TOXICITY DATA
MUTATION DATA
|
| Symbol |



GHS02, GHS06, GHS08 |
|---|---|
| Signal Word | Danger |
| Hazard Statements | H225-H301 + H311 + H331-H370 |
| Precautionary Statements | P210-P260-P280-P301 + P310-P311 |
| Personal Protective Equipment | dust mask type N95 (US);Eyeshields;Gloves |
| Hazard Codes | Xi: Irritant; |
| Risk Phrases | R36/37/38 |
| Safety Phrases | S24/25 |
| RIDADR | UN1230 - class 3 - PG 2 - Methanol, solution |
| WGK Germany | 2 |
| RTECS | BV8396000 |
| HS Code | 2937290090 |
| Precursor 8 | |
|---|---|
| DownStream 10 | |
| HS Code | 2937290014 |
|---|---|
| Summary | HS:2937290014 (3s,8r,9s,10r,13s,14s)-3-hydroxy-10,13-dimethyl-3,4,7,8,9,10,11,12,13,14,15,16-dodecahydro-1h-cyclopenta[a]phenanthren-17(2h)-one VAT:17.0% Tax rebate rate:9.0% Supervision conditions:l MFN tariff:4.0% General tariff:30.0% |
|
Assessment of the potential of polyphenols as a CYP17 inhibitor free of adverse corticosteroid elevation.
Biochem. Pharmacol. 90(3) , 288-96, (2014) Inhibition of 17α-hydroxylase/17,20-lyase (CYP17), which dictates the proceeding of androgen biosynthesis, is recommended as an effective treatment for androgen-dependent diseases. However, androgen d... |
|
|
Mechanistic Scrutiny Identifies a Kinetic Role for Cytochrome b5 Regulation of Human Cytochrome P450c17 (CYP17A1, P450 17A1).
PLoS ONE 10 , e0141252, (2015) Cytochrome P450c17 (P450 17A1, CYP17A1) is a critical enzyme in the synthesis of androgens and is now a target enzyme for the treatment of prostate cancer. Cytochrome P450c17 can exhibit either one or... |
|
|
Cheminformatics analysis of assertions mined from literature that describe drug-induced liver injury in different species.
Chem. Res. Toxicol. 23 , 171-83, (2010) Drug-induced liver injury is one of the main causes of drug attrition. The ability to predict the liver effects of drug candidates from their chemical structures is critical to help guide experimental... |
| 3β-Hydroxy-androst-5-ene-17-one |
| DHEA |
| 17-Hormoforin |
| 3-β-hydroxyandrost-5-en-17-one |
| 3b-hydroxy-Androst-5-en-17-one |
| (3b)-3-Hydroxyandrost-5-en-17-one |
| 3β-hydroxy-androst-5-en-17-one |
| (3β)-3-hydroxy-Androst-5-en-17-one |
| D5-Androsten-3b-ol-17-one |
| (3β)-3-Hydroxyandrost-5-en-17-one |
| MFCD00003613 |
| Psicosterone |
| Prasterone |
| trans-Dehydroandrosterone |
| 3b-Hydroxyandrost-5-ene-17-one |
| Androst-5-en-17-one, 3β-hydroxy- |
| Androst-5-en-17-one, 3-hydroxy-, (3β)- |
| 3b-Hydroxyandrost-5-en-17-one |
| Prestara |
| 3β-Hydroxy-5-androsten-17-one |
| Intrarosa |
| Deandros |
| dehydroisoandrosterone |
| (3β)-3-Hydroxyandrost-5-ene-17-one |
| Androst-5-ene-3b-ol-17-one |
| Androst-5-ene-3β-ol-17-one |
| 5-Androsten-3β-ol-17-one |
| Dehydro-epi-androsterone |
| 17-Chetovis |
| Dehydroepiandrosterone |
| didehydroepiandrosterone |
| 3β-Hydroxyandrost-5-ene-17-one |
| D5-Androsten-3β-ol-17-one |
| Diandrone |
| EINECS 200-175-5 |
| Astenile |
| 3β-Hydroxyandrost-5-en-17-one |
| UNII-459AG36T1B |
| Diandron |
 CAS#:853-23-6
CAS#:853-23-6![(17Ξ)-spiro[androst-5-en-17,2'-[1,3]oxathiolan]-3β-ol Structure](https://image.chemsrc.com/caspic/232/327622-68-4.png) CAS#:327622-68-4
CAS#:327622-68-4 CAS#:23549-24-8
CAS#:23549-24-8![3β-[2-(triphenylsilyl)ethoxycarbonyloxy]androst-5-en-17-one Structure](https://image.chemsrc.com/caspic/429/1310361-06-8.png) CAS#:1310361-06-8
CAS#:1310361-06-8 CAS#:18000-76-5
CAS#:18000-76-5 CAS#:145-13-1
CAS#:145-13-1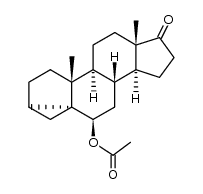 CAS#:24357-31-1
CAS#:24357-31-1 CAS#:81481-38-1
CAS#:81481-38-1![(1R,3S,5E)-5-[(2E)-2-[(3aS,7aS)-1-(4-hydroxy-4-methylpentoxy)-7a-methyl-2,3,3a,5,6,7-hexahydro-1H-inden-4-ylidene]ethylidene]-4-methylidenecyclohexane-1,3-diol structure](https://image.chemsrc.com/caspic/022/106315-28-0.png) CAS#:106315-28-0
CAS#:106315-28-0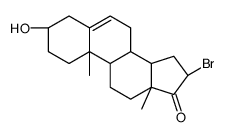 CAS#:1093-91-0
CAS#:1093-91-0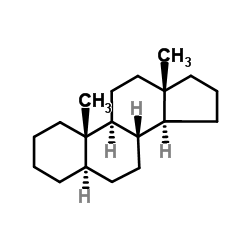 CAS#:438-22-2
CAS#:438-22-2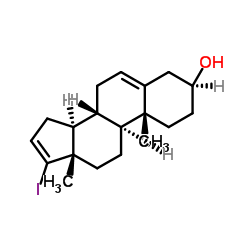 CAS#:32138-69-5
CAS#:32138-69-5 CAS#:5987-00-8
CAS#:5987-00-8 CAS#:510-64-5
CAS#:510-64-5![(10R,13S)-17-Chloro-16-formyl-10,13-dimethyl-2,3,4,7,8,9,10,11,12,13,14,15-dodecahydro-1H-cyclopenta[a]phenanthren-3-yl acetate structure](https://image.chemsrc.com/caspic/412/1865-56-1.png) CAS#:1865-56-1
CAS#:1865-56-1![10a,12a-dimethyl-2,3,3a,3b,4,5,8,9,10,10b,11,12-dodecahydroindeno[4,5-i][3]benzazepine-1,7-dione structure](https://image.chemsrc.com/caspic/104/20986-87-2.png) CAS#:20986-87-2
CAS#:20986-87-2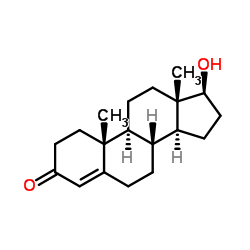 CAS#:58-22-0
CAS#:58-22-0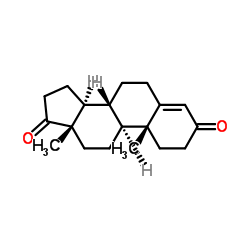 CAS#:63-05-8
CAS#:63-05-8
