| Description |
DL-Borneol is a racemic mixture of D-Borneol and L-Borneol. DL-Borneol is widely used for the treatment of cardiovascular and cerebrovascular diseases in China.
|
| Related Catalog |
|
| In Vitro |
DL-Borneol increases intracellular accumulation of Rho123, and enhances P-gp substrates across the BBB in vitro, and also depresses mdr1a mRNA and P-gp expression. Furthermore, DL-Borneol could activate NF-κB and inhibition of NF-κB with MG132 and SN50 obscures the P-gp decreases induced by DL-Borneol. 10 μg/mL and 20 μg/mL DL-Borneol significantly increase phosphorylation of IκB expression at 30 min after treatment transiently. DL-Borneol treatment decreases P-gp expression in BMECs[1].
|
| In Vivo |
DL-Borneol significantly suppresses the process of epileptogenesis in PTZ-kindled mice. The biochemical alterations induced by PTZ kindling are ameliorated in DL-Borneol-treated animals which is indicated by decreased LPO and increased SOD, GSH, CAT levels. The distinct neuronal damage observed in the kindled group is counteracted by DL-Borneol. Furthermore, it decreases the levels of GFAP which is manifested by reduced immunostaining[2]. The pathological damages of ischemia-reperfusion have a significant impact on the pharmacokinetic traits of DL-Borneol and that there are some components in Xingnaojing inhibiting the absorption of DL-Borneol[3].
|
| Cell Assay |
Rho123 efflux assay is used to measure the activity of P-gp in BMECs according to previous methods. BMECs grown to confluency in 24-well plates are treated with 5 μg/mL, 10 μg/mL and 20 μg/mL DL-Borneol, DMSO, CsA for 2 h, or with 10 μg/mL and 20 μg/mL DL-Borneol for different times (30 min, 1 h, 2 h, and 4 h). Then BMECs are exposed to 5 μmol/L Rho123 in DMEM for 90 min. After incubation with Rho123, BMECs are washed with ice-cold PBS and solubilized in 1% NaOH. Fluorescence of Rho123 is measured with emission wavelength at 535 nm and excitation wavelength at 485 nm using a fluorescence spectrophotometer[1].
|
| Animal Admin |
Rats: The pharmacokinetic study is performed 24 h after reperfusion in the model groups, i.e., 26 h after operation in the SO group. The XNJ subgroup rats are orally administered with XNJ decoction dissolved in 0.7% CMC-Na aqueous solution (10.00 ml/kg body weight (BW)). The pure DL-Borneol subgroup also receives gavages of DL-Borneol suspension (10.00 ml/kg BW). DL-Borneol and XNJ suspensions are administrated respectively at a dosage of 162.0 mg/kg of DL-Borneol. Then 0.5 ml plasma samples are collected into heparinized tubes by the puncture of the retro-orbital sinus at 5, 10, 20, 30, 45, 60, 90, 120, 180, 240, and 360 min separately following oral administration. After centrifugation at 6000 r/min for 10 min, plasma samples are stored at −20 °C and analyzed within one week[3]. Mice: Repeated administration of a subconvulsive dose of PTZ (35 mg/kg, i.p.) on every alternate day for 4 weeks produces kindling in mice. DL-Borneol (5, 10, and 25 mg/kg, i.p.) and diazepam (1 mg/kg, i.p.) are given as a pretreatment prior to each PTZ injection during the progression of kindling. Oxidative stress parameters such as superoxide dismutase (SOD), reduced glutathione (GSH), catalase (CAT), and lipid peroxidation (LPO) are assessed at the end of the study. Neuronal damage is assessed by hematoxylin and eosin staining technique. GFAP is also evaluated in the hippocampus region of the brain by using immunohistochemistry[2].
|
| References |
[1]. Fan X, et al. Borneol Depresses P-Glycoprotein Function by a NF-κB Signaling Mediated Mechanism in a Blood Brain Barrier in Vitro Model. Int J Mol Sci. 2015 Nov 18;16(11):27576-88. [2]. Tambe R, et al. Antiepileptogenic effects of borneol in pentylenetetrazole-induced kindling in mice. Naunyn Schmiedebergs Arch Pharmacol. 2016 May;389(5):467-75. [3]. Xu P, et al. Comparative pharmacokinetics of borneol in cerebral ischemia-reperfusion and sham-operated rats. J Zhejiang Univ Sci B. 2014 Jan;15(1):84-91.
|
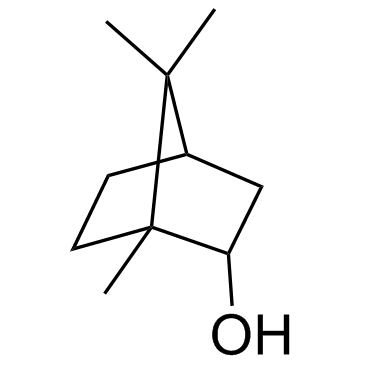
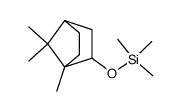 CAS#:74472-21-2
CAS#:74472-21-2![1,7,7-trimethyl-bicyclo[2.2.1]heptane-2-one Structure](https://image.chemsrc.com/caspic/166/736109-58-3.png) CAS#:736109-58-3
CAS#:736109-58-3 CAS#:75-09-2
CAS#:75-09-2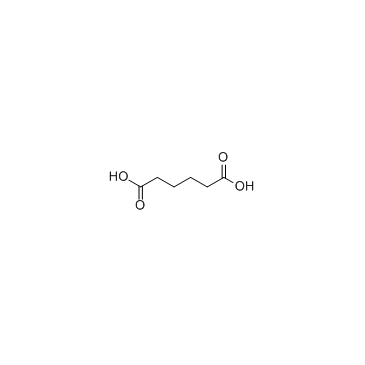 CAS#:124-04-9
CAS#:124-04-9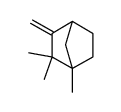 CAS#:13144-43-9
CAS#:13144-43-9 CAS#:76-22-2
CAS#:76-22-2 CAS#:79-92-5
CAS#:79-92-5 CAS#:64-17-5
CAS#:64-17-5 CAS#:6627-72-1
CAS#:6627-72-1 CAS#:60-29-7
CAS#:60-29-7 CAS#:565-00-4
CAS#:565-00-4 CAS#:124-83-4
CAS#:124-83-4 CAS#:464-49-3
CAS#:464-49-3 CAS#:76-49-3
CAS#:76-49-3 CAS#:142-29-0
CAS#:142-29-0![(4,7,7-trimethyl-3-bicyclo[2.2.1]heptanyl) pyridine-2-carboxylate structure](https://image.chemsrc.com/caspic/326/88382-43-8.png) CAS#:88382-43-8
CAS#:88382-43-8
