Epiandrosterone
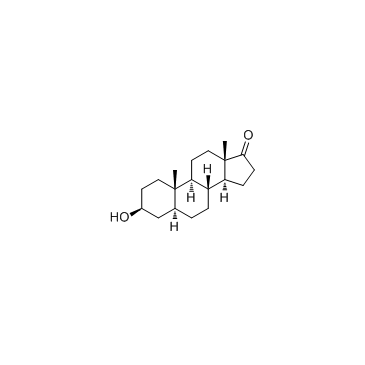
Epiandrosterone structure
|
Common Name | Epiandrosterone | ||
|---|---|---|---|---|
| CAS Number | 481-29-8 | Molecular Weight | 290.440 | |
| Density | 1.1±0.1 g/cm3 | Boiling Point | 413.1±45.0 °C at 760 mmHg | |
| Molecular Formula | C19H30O2 | Melting Point | 172-174 °C | |
| MSDS | USA | Flash Point | 176.4±21.3 °C | |
Use of EpiandrosteroneEpiandrosterone is a steroid hormone with weak androgenic activity. Epiandrosterone is naturally produced by the enzyme 5α-reductase from the adrenal hormone DHEA. |
| Name | epiandrosterone |
|---|---|
| Synonym | More Synonyms |
| Description | Epiandrosterone is a steroid hormone with weak androgenic activity. Epiandrosterone is naturally produced by the enzyme 5α-reductase from the adrenal hormone DHEA. |
|---|---|
| Related Catalog | |
| Target |
Human Endogenous Metabolite |
| References |
| Density | 1.1±0.1 g/cm3 |
|---|---|
| Boiling Point | 413.1±45.0 °C at 760 mmHg |
| Melting Point | 172-174 °C |
| Molecular Formula | C19H30O2 |
| Molecular Weight | 290.440 |
| Flash Point | 176.4±21.3 °C |
| Exact Mass | 290.224579 |
| PSA | 37.30000 |
| LogP | 3.75 |
| Vapour Pressure | 0.0±2.2 mmHg at 25°C |
| Index of Refraction | 1.536 |
| Water Solubility | practically insoluble |
| Personal Protective Equipment | Eyeshields;Gloves;type N95 (US);type P1 (EN143) respirator filter |
|---|---|
| Safety Phrases | S22-S24/25 |
| RIDADR | NONH for all modes of transport |
| WGK Germany | 3 |
| HS Code | 2937290090 |
| Precursor 9 | |
|---|---|
| DownStream 10 | |
| HS Code | 2937290090 |
|---|
|
Novel potent and selective bile acid derivatives as TGR5 agonists: biological screening, structure-activity relationships, and molecular modeling studies.
J. Med. Chem. 51 , 1831-41, (2008) TGR5, a metabotropic receptor that is G-protein-coupled to the induction of adenylate cyclase, has been recognized as the molecular link connecting bile acids to the control of energy and glucose home... |
|
|
Potent and selective steroidal inhibitors of 17beta-hydroxysteroid dehydrogenase type 7, an enzyme that catalyzes the reduction of the key hormones estrone and dihydrotestosterone.
J. Med. Chem. 52 , 7488-502, (2009) 17beta-Hydroxysteroid dehydrogenase type 7 (17beta-HSD7) catalyzes the reduction of estrone (E(1)) into estradiol (E(2)) and of dihydrotestosterone (DHT) into 5alpha-androstane-3beta,17beta-diol (3bet... |
|
|
A simple method for the small scale synthesis and solid-phase extraction purification of steroid sulfates.
Steroids 92 , 74-80, (2014) Steroid sulfates are a major class of steroid metabolite that are of growing importance in fields such as anti-doping analysis, the detection of residues in agricultural produce or medicine. Despite t... |
| UNII:8TR252Z538 |
| D-epiandrosterone |
| 3β-Hydroxyetioallocholan-17-one |
| 3b-Hydroxy-5a-androstane-17-one |
| 3β-Hydroxy-5α-androstan-17-one |
| 3BETA-HYDROXY-5ALPHA-ANDROSTAN-17-ONE |
| 3b-Androsterone |
| 3b-Androstanol-17-one |
| EINECS 207-563-3 |
| Epi-androsterone |
| 3β-hydroxy-androstan-17-one |
| ISOANDROSTERONE |
| MFCD00064134 |
| 5α-Androstan-17-one, 3β-hydroxy- |
| epiandrosteron |
| 3b-Hydroxy-17-oxo-5a-androstane |
| trans-Androsterone |
| Epiandrosterone |
| 3β-Androsterone |
| (3β,5α)-3-Hydroxyandrostan-17-one |
| 3B-HYDROXY-5A-ANDROSTAN-17-ONE |
| Androstan-17-one, 3-hydroxy-, (3β,5α)- |
| (3b,5a)-3-Hydroxyandrostan-17-one |
| (3S,5S,8R,9S,10S,13S,14S)-3-Hydroxy-10,13-dimethylhexadecahydro-17H-cyclopenta[a]phenanthren-17-one |
| Androsterone, (3β)- |
| 3b-Hydroxyetioallocholan-17-one |
| 3beta-Androsterone |
| EPLANDROSTERONE |
 CAS#:53-43-0
CAS#:53-43-0 CAS#:1239-31-2
CAS#:1239-31-2 CAS#:5615-34-9
CAS#:5615-34-9 CAS#:1044-89-9
CAS#:1044-89-9 CAS#:846-46-8
CAS#:846-46-8 CAS#:571-20-0
CAS#:571-20-0 CAS#:102698-33-9
CAS#:102698-33-9![(3S,5S,8R,9S,10S,13S,14S)-10,13-dimethyl-3-((tetrahydrofuran-2-yl)oxy)tetradecahydro-1H-cyclopenta[a]phenanthren-17(2H)-one Structure](https://image.chemsrc.com/caspic/265/111237-01-5.png) CAS#:111237-01-5
CAS#:111237-01-5 CAS#:1156-92-9
CAS#:1156-92-9 CAS#:14639-79-3
CAS#:14639-79-3![(5S,8R,9S,10S,13S,14S)-10,13-dimethyl-1,2,5,6,7,8,9,11,12,14,15,16-dodecahydrocyclopenta[a]phenanthren-17-one structure](https://image.chemsrc.com/caspic/321/14935-81-0.png) CAS#:14935-81-0
CAS#:14935-81-0 CAS#:2429-15-4
CAS#:2429-15-4 CAS#:53-41-8
CAS#:53-41-8![(3S,5R,8R,9S,10S,13S,14S,17R)-10,13-dimethyl-2,3,4,5,6,7,8,9,11,12,14,15,16,17-tetradecahydro-1H-cyclopenta[a]phenanthrene-3,17-diol structure](https://image.chemsrc.com/caspic/153/5856-11-1.png) CAS#:5856-11-1
CAS#:5856-11-1 CAS#:6247-88-7
CAS#:6247-88-7 CAS#:138313-21-0
CAS#:138313-21-0 CAS#:601-08-1
CAS#:601-08-1
