Methotrexate-d3

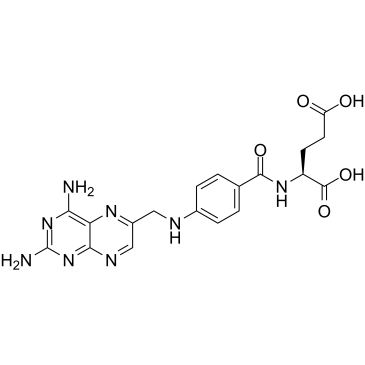
Methotrexate-d3 structure
|
Common Name | Methotrexate-d3 | ||
|---|---|---|---|---|
| CAS Number | 432545-63-6 | Molecular Weight | 457.45800 | |
| Density | N/A | Boiling Point | N/A | |
| Molecular Formula | C20H19D3N8O5 | Melting Point | 210°C dec. | |
| MSDS | N/A | Flash Point | 9℃ | |
| Symbol |



GHS02, GHS06, GHS08 |
Signal Word | Danger | |
Use of Methotrexate-d3Methotrexate-d3 (Amethopterin-d3) is the deuterium labeled Methotrexate. Methotrexate (Amethopterin), an antimetabolite and antifolate agent, inhibits the enzyme dihydrofolate reductase, thereby preventing the conversion of folic acid into tetrahydrofolate, and inhibiting DNA synthesis. Methotrexate, also an immunosuppressant and antineoplastic agent, is used for the research of rheumatoid arthritis and a number of different cancers (such as acute lymphoblastic leukemia)[1][2][3]. |
| Name | methotrexate-d3 |
|---|
| Description | Methotrexate-d3 (Amethopterin-d3) is the deuterium labeled Methotrexate. Methotrexate (Amethopterin), an antimetabolite and antifolate agent, inhibits the enzyme dihydrofolate reductase, thereby preventing the conversion of folic acid into tetrahydrofolate, and inhibiting DNA synthesis. Methotrexate, also an immunosuppressant and antineoplastic agent, is used for the research of rheumatoid arthritis and a number of different cancers (such as acute lymphoblastic leukemia)[1][2][3]. |
|---|---|
| Related Catalog | |
| In Vitro | Stable heavy isotopes of hydrogen, carbon, and other elements have been incorporated into drug molecules, largely as tracers for quantitation during the drug development process. Deuteration has gained attention because of its potential to affect the pharmacokinetic and metabolic profiles of drugs[1]. |
| References |
[3]. Swierkot J, et al. Methotrexate in rheumatoid arthritis. Pharmacol Rep. 2006 Jul-Aug;58(4):473-92. |
| Melting Point | 210°C dec. |
|---|---|
| Molecular Formula | C20H19D3N8O5 |
| Molecular Weight | 457.45800 |
| Flash Point | 9℃ |
| Exact Mass | 457.19000 |
| PSA | 210.54000 |
| LogP | 1.82170 |
| InChIKey | FBOZXECLQNJBKD-FUPFOCIHSA-N |
| SMILES | CN(Cc1cnc2nc(N)nc(N)c2n1)c1ccc(C(=O)NC(CCC(=O)O)C(=O)O)cc1 |
| Storage condition | -20°C Freezer |
| Symbol |



GHS02, GHS06, GHS08 |
|---|---|
| Signal Word | Danger |
| Hazard Statements | H225-H301 + H311 + H331-H370 |
| Precautionary Statements | P210-P260-P280-P301 + P310 + P330-P308 + P311-P403 + P233 |
| Hazard Codes | F,T |
| Risk Phrases | 11-23/24/25-39/23/24/25 |
| Safety Phrases | 7-16-36/37-45 |
| RIDADR | UN1230 - class 3 - PG 2 - Methanol, solution |
|
~% 
Methotrexate-d3 CAS#:432545-63-6 |
| Literature: Elmore, Charles S.; Dean, Dennis C.; Zhang, Yong; Gibson, Carter; Jenkins, Herb; Jones, Allen N.; Melillo, David G. Journal of Labelled Compounds and Radiopharmaceuticals, 2002 , vol. 45, # 1 p. 29 - 36 |
| Precursor 2 | |
|---|---|
| DownStream 1 | |
|
A surface wipe sampling and LC-MS/MS method for the simultaneous detection of six antineoplastic drugs commonly handled by healthcare workers.
Anal. Bioanal. Chem 407 , 7083-92, (2015) An effective wipe sampling and LC-MS/MS method was developed to simultaneously analyze six commonly administered antineoplastic drugs in stainless steel surface. The analyzed drugs were methotrexate, ... |
![dimethyl 2-[[4-[(2,4-diaminopteridin-6-yl)methyl-(trideuteriomethyl)amino]benzoyl]amino]pentanedioate structure](https://image.chemsrc.com/caspic/178/432545-60-3.png) CAS#:432545-60-3
CAS#:432545-60-3