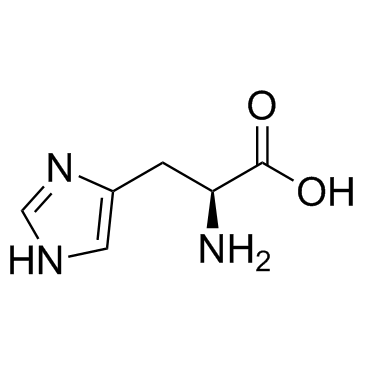
L-Histidine, N-formyl-
Modify Date: 2025-08-26 11:57:58
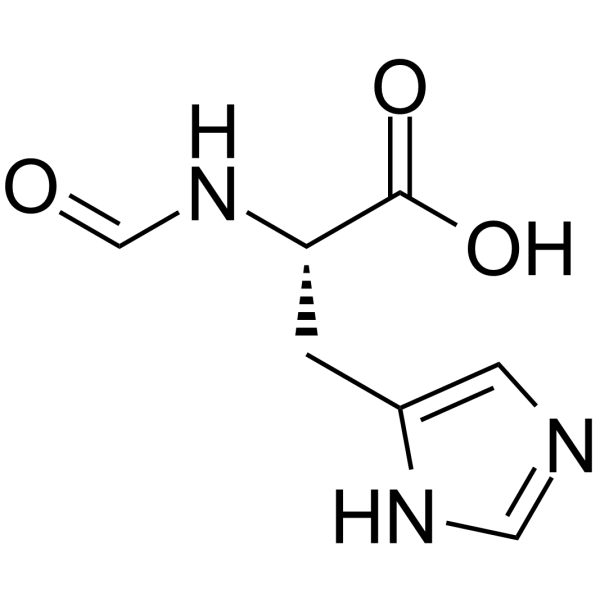
L-Histidine, N-formyl- structure
|
Common Name | L-Histidine, N-formyl- | ||
|---|---|---|---|---|
| CAS Number | 15191-21-6 | Molecular Weight | 183.16500 | |
| Density | 1.51g/cm3 | Boiling Point | 442.9ºC at 760mmHg | |
| Molecular Formula | C7H9N3O3 | Melting Point | 202.0 to 206.0 °C | |
| MSDS | USA | Flash Point | 221.6ºC | |
Use of L-Histidine, N-formyl-N-Formyl-L-histidine shows binding affinity to histidyl-tRNA synthetase with a Ki value of 4.6 μM. N-Formyl-L-histidine shows a competitive inhibition against L-histidine ammonia-lyase, inhibits urocanic acid formation from L-histidine with a Ki value of 4.26 mM[1][2]. |
| Name | n-formyl-l-histidine |
|---|---|
| Synonym | More Synonyms |
| Description | N-Formyl-L-histidine shows binding affinity to histidyl-tRNA synthetase with a Ki value of 4.6 μM. N-Formyl-L-histidine shows a competitive inhibition against L-histidine ammonia-lyase, inhibits urocanic acid formation from L-histidine with a Ki value of 4.26 mM[1][2]. |
|---|---|
| Related Catalog | |
| References |
| Density | 1.51g/cm3 |
|---|---|
| Boiling Point | 442.9ºC at 760mmHg |
| Melting Point | 202.0 to 206.0 °C |
| Molecular Formula | C7H9N3O3 |
| Molecular Weight | 183.16500 |
| Flash Point | 221.6ºC |
| Exact Mass | 183.06400 |
| PSA | 95.08000 |
| LogP | 0.17820 |
| Vapour Pressure | 1.72E-17mmHg at 25°C |
| Index of Refraction | 58 ° (C=2, H2O) |
| Water Solubility | almost transparency |
| Safety Phrases | S22-S24/25 |
|---|---|
| HS Code | 2933290090 |
|
~% 
L-Histidine, N-... CAS#:15191-21-6 |
| Literature: Justus Liebigs Annalen der Chemie, , vol. 363, p. 116 |
| Precursor 2 | |
|---|---|
| DownStream 0 | |
| HS Code | 2933290090 |
|---|---|
| Summary | 2933290090. other compounds containing an unfused imidazole ring (whether or not hydrogenated) in the structure. VAT:17.0%. Tax rebate rate:13.0%. . MFN tariff:6.5%. General tariff:20.0% |
| (S)-2-Formamido-3-(1H-imidazol-4-yl)propanoic acid |
| N-Formyl-L-histidine |
| N-formyl histidine |
| Einecs 239-248-1 |
| Formylhistidine |
| FOR-HIS-OH |