Risperidone

Risperidone structure
|
Common Name | Risperidone | ||
|---|---|---|---|---|
| CAS Number | 106266-06-2 | Molecular Weight | 410.484 | |
| Density | 1.4±0.1 g/cm3 | Boiling Point | 572.4±60.0 °C at 760 mmHg | |
| Molecular Formula | C23H27FN4O2 | Melting Point | 170°C | |
| MSDS | Chinese USA | Flash Point | 300.0±32.9 °C | |
| Symbol |



GHS02, GHS06, GHS08 |
Signal Word | Danger | |
Use of RisperidoneRisperidone is a serotonin 5-HT2 receptor blocker, P-Glycoprotein inhibitor and potent dopamine D2 receptor antagonist, with Kis of 4.8, 5.9 nM for 5-HT2A and dopamine D2 receptor, respectively. |
| Name | risperidone |
|---|---|
| Synonym | More Synonyms |
| Description | Risperidone is a serotonin 5-HT2 receptor blocker, P-Glycoprotein inhibitor and potent dopamine D2 receptor antagonist, with Kis of 4.8, 5.9 nM for 5-HT2A and dopamine D2 receptor, respectively. |
|---|---|
| Related Catalog | |
| Target |
5-HT2A Receptor:4.8 nM (Ki) dopamine D2 receptor:5.9 nM (Ki) P-Glycoprotein |
| In Vitro | Risperidone is a serotonin 5-HT2 receptor blocker, P-Glycoprotein inhibitor and potent dopamine D2 receptor antagonist, with Kis of 4.8, 5.9 nM for 5-HT2A and dopamine D2 receptor, respectively. Risperidone dose-dependently inhibited the release of IL-12 in mature DCs, while the production of IL-10 is dose-dependently increased by Risperidone. A high dose of risperidone can induce TNF-α release from mature DCs[3]. |
| In Vivo | In the first experiment, body weight is found to be slightly but significantly lower in the Risperidone-treated rats as a function of age. Similar to the first experiment, age-dependent differences in body weight are also observed between the three treatment groups in the second locomotor experiment. Rats treated with the 3.0 mg/kg dose of Risperidone weigh less than vehicle-treated rats on postnatal days 35, 38, and 41. The third locomotor experiment involves larger, mixed-sex litters in contrast to the smaller, single-sex litters used in the first two experiments. As noted for the first two experiments, rats treated with Risperidone in the third experiment gain less weight in an age-dependent manner[4]. |
| Animal Admin | Rats[4] A total of 211 Long-Evans rats (56 females and 155 males) are used. Within each study, three groups of roughly equal numbers of rats receive injections of 1.0 mg/kg of Risperidone, 3.0 mg/kg of Risperidone, or the vehicle used for the Risperidone solution as a control. In the first experiment, twenty-six male rats (n=9 in the vehicle and 3.0 mg/kg Risperidone groups; n=8 in the 1.0 mg/kg Risperidone group) are tested for locomotor activity for 20 minutes a day beginning at postnatal day 49 and continuing daily until postnatal day 53. A second experiment determined if the locomotor effects of early-life Risperidone treatment persisted well into adulthood. A third experiment ascertains the effects of sex on the locomotor effects of early-life Risperidone seen in young adult rats. In this experiment, sixty male (n=20 per treatment group) and 56 female (n=19 rats in the vehicle and 3.0 mg/kg dose group, n=18 in the 1.0 mg/kg dose group) rats are treated. A fourth experiment assessed reversal learning during adulthood in rats administered earlylife risperidone. Forty-two male rats (n=14 per treatment group) are treated[4]. |
| References |
| Density | 1.4±0.1 g/cm3 |
|---|---|
| Boiling Point | 572.4±60.0 °C at 760 mmHg |
| Melting Point | 170°C |
| Molecular Formula | C23H27FN4O2 |
| Molecular Weight | 410.484 |
| Flash Point | 300.0±32.9 °C |
| Exact Mass | 410.211792 |
| PSA | 64.16000 |
| LogP | 2.88 |
| Vapour Pressure | 0.0±1.6 mmHg at 25°C |
| Index of Refraction | 1.677 |
| Storage condition | Room temp |
CHEMICAL IDENTIFICATION
HEALTH HAZARD DATAACUTE TOXICITY DATA
|
| Symbol |



GHS02, GHS06, GHS08 |
|---|---|
| Signal Word | Danger |
| Hazard Statements | H225-H301 + H311 + H331-H370 |
| Precautionary Statements | P210-P260-P280-P301 + P310-P311 |
| Personal Protective Equipment | Eyeshields;Faceshields;Gloves;type P2 (EN 143) respirator cartridges |
| Hazard Codes | T: Toxic; |
| Risk Phrases | R25 |
| Safety Phrases | S28-S36-S45 |
| RIDADR | 3249 |
| RTECS | UV1164800 |
| Packaging Group | II |
| Hazard Class | 6.1(a) |
| HS Code | 2934999090 |
| Precursor 9 | |
|---|---|
| DownStream 3 | |
| HS Code | 2934999090 |
|---|---|
| Summary | 2934999090. other heterocyclic compounds. VAT:17.0%. Tax rebate rate:13.0%. . MFN tariff:6.5%. General tariff:20.0% |
|
Nest building is impaired in the Ts65Dn mouse model of Down syndrome and rescued by blocking 5HT2a receptors.
Neurobiol. Learn. Mem. 116 , 162-71, (2014) Down syndrome (DS) has an incidence of about 1/700 births, and is therefore the most common cause of cognitive and behavioral impairments in children. Recent studies on mouse models of DS indicate tha... |
|
|
Brexpiprazole I: in vitro and in vivo characterization of a novel serotonin-dopamine activity modulator.
J. Pharmacol. Exp. Ther. 350(3) , 589-604, (2014) Brexpiprazole (OPC-34712, 7-{4-[4-(1-benzothiophen-4-yl)piperazin-1-yl]butoxy}quinolin-2(1H)-one) is a novel drug candidate in clinical development for psychiatric disorders with high affinity for ser... |
|
|
Utility of cerebrospinal fluid drug concentration as a surrogate for unbound brain concentration in nonhuman primates.
Drug Metab. Pharmacokinet. 29(5) , 419-26, (2014) In central nervous system drug discovery, cerebrospinal fluid (CSF) drug concentration (C(CSF)) has been widely used as a surrogate for unbound brain concentrations (C(u,brain)). However, previous rod... |
| Rispadal |
| Psychodal |
| 3-{2-[4-(6-Fluoro-1,2-benzoxazol-3-yl)-1-piperidinyl]ethyl}-2-methyl-6,7,8,9-tetrahydro-4H-pyrido[1,2-a]pyrimidin-4-one |
| 3-{2-[4-(6-Fluor-1,2-benzisoxazol-3-yl)piperidin-1-yl]ethyl}-2-methyl-6,7,8,9-tetrahydro-4H-pyrido[1,2-a]pyrimidin-4-on |
| MFCD00274576 |
| Belivon |
| Risperidal |
| Kesperidone |
| Risperdal |
| Spiron |
| 3-{2-[4-(6-fluoro-1,2-benzisoxazol-3-yl)pipéridin-1-yl]éthyl}-2-méthyl-6,7,8,9-tétrahydro-4H-pyrido[1,2-a]pyrimidin-4-one |
| 3-{2-[4-(6-fluoro-1,2-benzoxazol-3-yl)piperidin-1-yl]ethyl}-2-methyl-6,7,8,9-tetrahydro-4H-pyrido[1,2-a]pyrimidin-4-one |
| Risperidone |
| Apexidone |
| R 64 766,Risperdal |
| 4H-Pyrido[1,2-a]pyrimidin-4-one, 3-[2-[4-(6-fluoro-1,2-benzisoxazol-3-yl)-1-piperidinyl]ethyl]-6,7,8,9-tetrahydro-2-methyl- |
| R 64,766,Risperidone |
![3-{2-[4-(6-fluorobenzo[d]isoxazol-3-yl)-piperidin-1-yl]-vinyl}-2-methyl-6,7,8,9-tetrahydro-pyrido[1,2-a]pyrimidin-4-one Structure](https://image.chemsrc.com/caspic/017/599173-41-8.png) CAS#:599173-41-8
CAS#:599173-41-8![3-(2-Chloroethyl)-6,7,8,9-tetrahydro-2-methyl-4H-pyrido[1,2-a]pyrimidin-4-one Structure](https://image.chemsrc.com/caspic/190/63234-80-0.png) CAS#:63234-80-0
CAS#:63234-80-0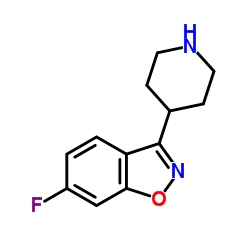 CAS#:84163-77-9
CAS#:84163-77-9![3-(2-(4-((2,4-difluorophenyl)(hydroxyimino)methyl)piperidin-1-yl)ethyl)-2-methyl-6,7,8,9-tetrahydro-4H-pyrido[1,2-a]pyrimidin-4-one Structure](https://image.chemsrc.com/caspic/482/158697-66-6.png) CAS#:158697-66-6
CAS#:158697-66-6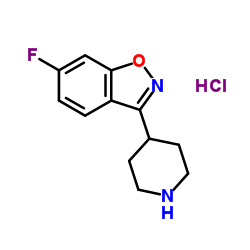 CAS#:84163-13-3
CAS#:84163-13-3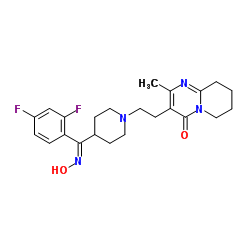 CAS#:132961-05-8
CAS#:132961-05-8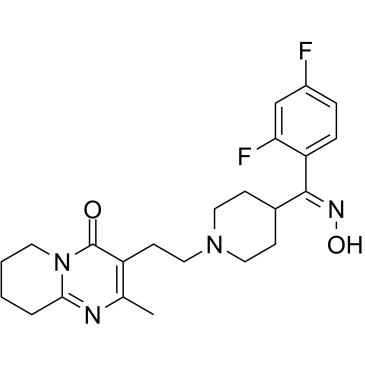 CAS#:691007-09-7
CAS#:691007-09-7![2,3,5,6-tetrabromocyclohexa-2,5-diene-1,4-dione compound with 3-(2-(4-(6-fluorobenzo[d]isoxazol-3-yl)piperidin-1-yl)ethyl)-2-methyl-6,7,8,9-tetrahydro-4H-pyrido[1,2-a]pyrimidin-4-one (1:1) Structure](https://image.chemsrc.com/caspic/497/1425681-48-6.png) CAS#:1425681-48-6
CAS#:1425681-48-6![4,5-dichloro-3,6-dioxocyclohexa-1,4-diene-1,2-dicarbonitrile compound with 3-(2-(4-(6-fluorobenzo[d]isoxazol-3-yl)piperidin-1-yl)ethyl)-2-methyl-6,7,8,9-tetrahydro-4H-pyrido[1,2-a]pyrimidin-4-one (1:1) Structure](https://image.chemsrc.com/caspic/111/1425681-45-3.png) CAS#:1425681-45-3
CAS#:1425681-45-3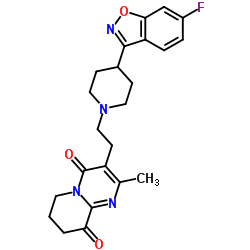 CAS#:1189516-65-1
CAS#:1189516-65-1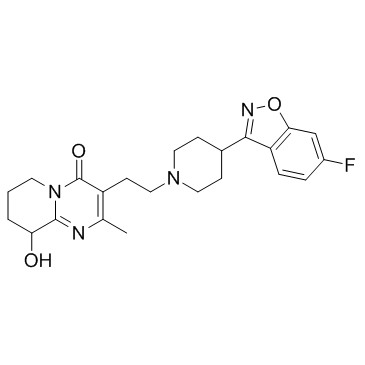 CAS#:144598-75-4
CAS#:144598-75-4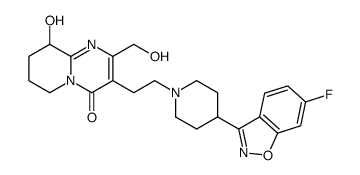 CAS#:1301724-94-6
CAS#:1301724-94-6