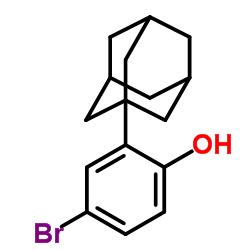
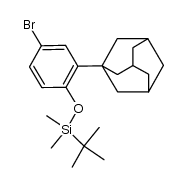
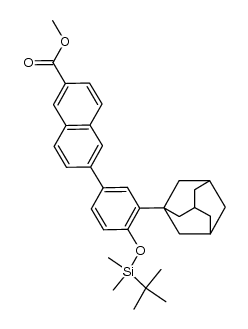
125316-60-1
| Name | 6-[3-(1-adamantyl)-4-hydroxyphenyl]naphthalene-2-carboxylic acid |
|---|---|
| Synonyms |
6-[3-(Adamantan-1-yl)-4-hydroxyphenyl]-2-naphthoic acid
O-Desmethyl Adapalene 6-[3-(1-Adamantyl)-4-hydroxyphenyl]-2-naphthalene carboxylic acid AHPN AHPN 6-[4-hydroxy-3-(tricyclo[3.3.1.1]dec-1-yl)phenyl]naphthalene-2-carboxylic acid Cd 437 CD437 |
| Description | CD437 is a selective Retinoic Acid Receptor γ (RARγ) agonist. |
|---|---|
| Related Catalog | |
| Target |
Retinoic Acid Receptor γ (RARγ)[1] |
| In Vitro | CD437 is a selective RARγ agonist. Growth inhibition by CD437 in these lung cancer cell lines is apparent after 2 days of treatment with 10 μM CD437. Dose-response experiments demonstrate that CD437 reduces the numbers of H460, SK-MES-1, A549, and H292 cells with 50% inhibitory values of approximately 0.5, 0.4, 3, and 0.85 μM, respectively[1]. Treatment for 72 h with CD437 causes a strong dose-dependent growth inhibition in all melanoma cell lines. At a concentration of 5 μM CD437, only about 5 to 25% of the cells remain viable after 3 d. The concentrations of CD437 required for 50% growth inhibition (IC50) range from 10 μM for MeWo to 0.1 μM for SK-Mel-23 showing the highest sensitivity[2]. |
| In Vivo | Tumors in CD437-treated mice stop growing, an effect that becomes already statistically significant (P<0.01) at day 13, 3 d after first administration of CD437, and is maintained for more than 3 wk after discontinuation of treatment. Further histologic analysis demonstrates marked c-fos mRNA levels at the tumor-stroma edge in CD437-treated tumors[2]. |
| Cell Assay | For morphological analysis, cells are treated with 10 μM CD437, trypsinized, washed with phosphate-buffered saline (PBS), fixed with 3.7% paraformaldehyde, and stained with 50 μg of 4,6-diamidino-2-phenylindole (DAPI) per mL containing 100 μg of DNase-free RNase A per mL to visualize the nuclei. Stained cells are examined by fluorescence microscopy. For the terminal deoxynucleotidyl transferase (TdT) assay, cells are treated with or without 10 μM CD437. After treatment, cells are trypsinized, washed with PBS, fixed in 1% formaldehyde in PBS, washed with PBS, resuspended in 70% ice-cold ethanol, and immediately stored at -20°C overnight. Cells are then labeled with biotin-16-dUTP by terminal transferase and stained with avidin-FITC (fluorescein isothiocyanate). The labeled cells are analyzed with a flow cytometer[1]. |
| Animal Admin | Male Swiss-nu/nu mice weighing 20 to 25 g are used in this study. Mice are kept under sterile conditions at 24 to 26°C room temperature, 50% relative humidity, and 12 h light-dark rhythm in laminar flow shelves and are supplied with autoclaved food and bedding. For treatment of melanoma xenografts, previously established MeWo melanoma tumors of 1 to 2 mm in diameter are implanted into the right flank of animals. After tumor growth for 10 d, groups of mice (n=8) are either treated with saline p.o. or are injected intratumorally for 3 wk or are fed with various concentrations of CD437 (10 mg/kg/body weight and 30 mg/kg/body weight). In addition, tumors of a fifth group are injected with CD437 (10 mg/kg/body weight) each day. Mice are visited daily and growing tumors are measured twice weekly with a caliperlike instrument[2]. |
| References |
| Density | 1.3±0.1 g/cm3 |
|---|---|
| Boiling Point | 595.0±50.0 °C at 760 mmHg |
| Melting Point | 271.6-276ºC |
| Molecular Formula | C27H26O3 |
| Molecular Weight | 398.493 |
| Flash Point | 327.7±26.6 °C |
| Exact Mass | 398.188202 |
| PSA | 57.53000 |
| LogP | 7.45 |
| Vapour Pressure | 0.0±1.8 mmHg at 25°C |
| Index of Refraction | 1.689 |
| Storage condition | -20°C |
CHEMICAL IDENTIFICATION
HEALTH HAZARD DATAACUTE TOXICITY DATA
|
| Personal Protective Equipment | Eyeshields;Gloves;type N95 (US);type P1 (EN143) respirator filter |
|---|---|
| Safety Phrases | S22 |
| RIDADR | NONH for all modes of transport |
| WGK Germany | 3 |
| RTECS | QJ1975900 |
|
~76% 
125316-60-1 |
| Literature: Charpentier; Bernardon; Eustache; Millois; Martin; Michel; Shroot Journal of Medicinal Chemistry, 1995 , vol. 38, # 26 p. 4993 - 5006 |
|
~% 
125316-60-1 |
| Literature: Journal of Medicinal Chemistry, , vol. 38, # 26 p. 4993 - 5006 |
|
~% 
125316-60-1 |
| Literature: Journal of Medicinal Chemistry, , vol. 38, # 26 p. 4993 - 5006 |
|
~% 
125316-60-1 |
| Literature: Journal of Medicinal Chemistry, , vol. 38, # 26 p. 4993 - 5006 |
|
~% 
125316-60-1 |
| Literature: Journal of Medicinal Chemistry, , vol. 38, # 26 p. 4993 - 5006 |
|
~% 
125316-60-1 |
| Literature: Journal of Medicinal Chemistry, , vol. 38, # 26 p. 4993 - 5006 |
| Precursor 6 | |
|---|---|
| DownStream 0 | |