317318-70-0
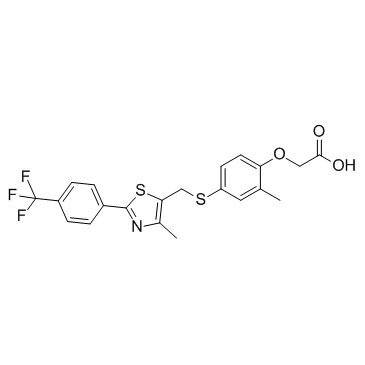
| Name | 2-[2-methyl-4-[[4-methyl-2-[4-(trifluoromethyl)phenyl]-1,3-thiazol-5-yl]methylsulfanyl]phenoxy]acetic acid |
|---|---|
| Synonyms |
GW 501516
2-(4-((2-(4-(Trifluoromethyl)phenyl)-5-methylthiazol-4-yl)methylthio)-2-methylphenoxy)acetic acid 2-(2-BROMOPHENYL)-3'-TRIFLUOROMETHYLACETOPHENONE 2-methyl-4-(((4-methyl-2-(4-(trifluoromethyl)phenyl)-5-(thiazolyl)methyl)thio)phenoxy)acetic acid Endurobol {2-Methyl-4-{{4-methyl-2-[4-(trifluoromethyl)phenyl]-5-thiazolyl}methylthio}phenoxy}acetic acid UNII-7I2HA1NU22 GSK-516 Cardarine GW-501516 GW501516 |
| Description | GW 501516 is a PPARδ agonist with an EC50 of 1.1 nM. |
|---|---|
| Related Catalog | |
| Target |
PPARδ:1.1 nM (EC50) |
| In Vitro | GW 501516 is shown to be the most potent and selective PPARα agonists known with an EC50 of 1.1 nM against PPARα and 1000-fold selectivity over the other human subtypes, PPARα and-γ[1]. GW 501516 exerts anti-inflammatory effects in mouse cultured proximal tubular (mProx) cells. GW 501516 inhibits palmitate- and TNFα-induced increases in MCP-1 mRNA expression in a dose-dependent manner[3]. |
| In Vivo | GW 501516 causes impaired bone formation, leading to decreased BMD and deterioration of bone properties in OVX rats[2]. GW 501516 attenuates interstitial inflammation and proximal tubular cell damage in a protein-overload mouse nephropathy model[3]. GW 501516 treatment enhances running endurance and the proportion of succinate dehydrogenase (SDH)-positive muscle fibres in both trained and untrained mice[4]. |
| Cell Assay | GW 501516 is dissolved in DMSO. Cells are starved by incubation in 0.2% FCS DMEM for 9 h, then pre-incubated with GW 501516, at a final concentration of 2.5 and 5 µM, or 0.05% DMSO as control for 3 hours, followed by stimulation with 150 µM palmitate bound to 8.0% BSA for 12 h[3]. |
| Animal Admin | Rats: Female Sprague Dawley rats, 12 weeks of age, are allocated to a sham-operated group and 3 OVX groups; high-dose GW 501516 (OVX-GW5), low-dose GW 501516 (OVX-GW1), and a control group (OVX-CTR), respectively. Animals receive GW 501516 or vehicle (methylcellulose) daily for 4 months by gavage. Bone mineral density (BMD) is assessed by dual x-ray absorptiometry at the femur, spine, and whole body[2]. Mice: Mice are randomly allocated to different groups and receive therapeutic diet and treatment. The GW 501516-containing rodent diet is made by evenly adding GW 501516 to the control diet to a final concentration of 0.04% w/w. In the control diet, 10% of the total calories are from fat (5.5% from soybean oil and 4.5% from lard)[3]. |
| References |
| Density | 1.4±0.1 g/cm3 |
|---|---|
| Boiling Point | 584.5±60.0 °C at 760 mmHg |
| Melting Point | 134-136°C |
| Molecular Formula | C21H18F3NO3S2 |
| Molecular Weight | 453.498 |
| Flash Point | 307.3±32.9 °C |
| Exact Mass | 453.068024 |
| PSA | 112.96000 |
| LogP | 6.29 |
| Vapour Pressure | 0.0±1.7 mmHg at 25°C |
| Index of Refraction | 1.619 |
| Storage condition | Refrigerator |
| Stability | Light Sensitive |
| Water Solubility | DMSO: soluble20mg/mL, clear |
| Hazard Codes | Xi |
|---|---|
| RIDADR | NONH for all modes of transport |
| RTECS | AI9105500 |