481-53-8
| Name | tangeretin |
|---|---|
| Synonyms |
Ponkanetin
4',5,6,7,8-pentamethoxyflavone Flavone, 5,6,7,8,4'-pentamethoxy TANGERETIN,NATURAL MFCD00017438 5,6,7,8,4'-Pentamethoxyflavone 5,6,7,8-Tetramethoxy-2-(4-methoxyphenyl)-4H-chromen-4-one TANGARETIN Tangeritin Tangeritine EINECS 207-570-1 Tangeretin 5-18-05-00491 (Beilstein Handbook Reference) |
| Description | Tangeretin, a flavonoid from citrus fruit peels, has been proven to play an important role in anti-inflammatory responses and neuroprotective effects in several disease models, and was also selected as a Notch-1 inhibitor.IC50 value:Target: Notch-1In vitro: Tangeretin enhanced the radiosensitivity of GC cells as demonstrated by MTT and colony formation assays. Tangeretin also attenuated radiation-induced EMT, invasion and migration in GC cells, accompanied by a decrease in Notch-1, Jagged1/2, Hey-1 and Hes-1 expressions. Tangeretin triggered the upregulation of miR-410, a tumor-suppressive microRNA. Furthermore, re-expression of miR-410 prevented radiation-induced EMT and cell invasion [1]. In vivo: In this study, we investigated the in vivo anti-RSV activity of tangeretin in 3-week-old male BALB/c mice. A plaque reduction assay and fluorescence quantitative polymerase chain reaction (FQ-PCR) showed that tangeretin inhibited RSV replication in the lung of mice [2]. |
|---|---|
| Related Catalog | |
| References |
| Density | 1.2±0.1 g/cm3 |
|---|---|
| Boiling Point | 565.3±50.0 °C at 760 mmHg |
| Melting Point | 155 °C |
| Molecular Formula | C20H20O7 |
| Molecular Weight | 372.369 |
| Flash Point | 248.4±30.2 °C |
| Exact Mass | 372.120911 |
| PSA | 76.36000 |
| LogP | 2.66 |
| Vapour Pressure | 0.0±1.5 mmHg at 25°C |
| Index of Refraction | 1.566 |
| Water Solubility | insoluble |
CHEMICAL IDENTIFICATION
HEALTH HAZARD DATAACUTE TOXICITY DATA
|
| Symbol |

GHS06 |
|---|---|
| Signal Word | Danger |
| Hazard Statements | H300 |
| Precautionary Statements | P264-P301 + P310 |
| Personal Protective Equipment | Eyeshields;Faceshields;Gloves;type P2 (EN 143) respirator cartridges |
| Hazard Codes | T:Toxic; |
| Risk Phrases | R25 |
| Safety Phrases | S45 |
| RIDADR | UN 2811 |
| RTECS | DJ3102725 |
| Hazard Class | 6.1 |
| Precursor 8 | |
|---|---|
| DownStream 8 | |




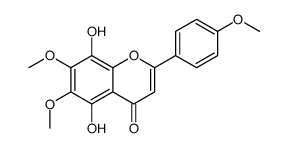

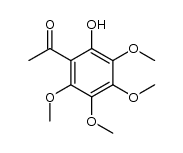
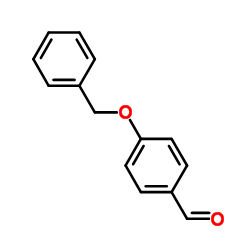

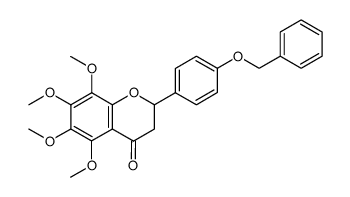


![4H-1-Benzopyran-4-one,2-[4-(acetyloxy)phenyl]-5,6,7,8-tetramethoxy structure](https://image.chemsrc.com/caspic/488/6959-55-3.png)
