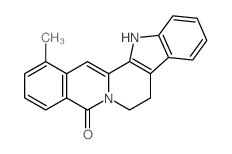
146-48-5
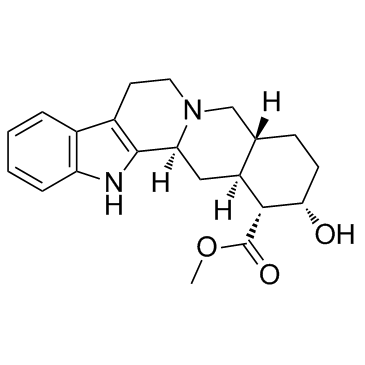
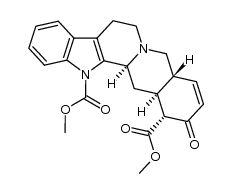
| Name | yohimbine |
|---|---|
| Synonyms |
[3H]-Yohimbine
Aphrosol Methyl (16α,17α)-17-hydroxyyohimban-16-carboxylate Yohimban-16α-carboxylic acid, 17α-hydroxy-, methyl ester (8CI) corynine yohimbine (16α,17α)-17-Hydroxy-yohimban-16-carboxylic acid methyl ester quebrachine Quebrachin Yohimbine HCl (16a,17a)-17-Hydroxyyohimban-16-carboxylic Acid Methyl Ester Yohimex Yocon APHRODINE methyl (1S,15R,18S,19R,20S)-18-hydroxy-1,3,11,12,14,15,16,17,18,19,20,21-dodecahydroyohimban-19-carboxylate MFCD00042748 Yohimban-16-α-carboxylic acid, 17-α-hydroxy-, methyl ester EINECS 205-672-0 (+)-Yohimbin Aphrodyne Yohimbin |
| Description | Yohimbine is a potent and relatively nonselective alpha 2-adrenergicreceptor (AR) antagonist, with IC50 of 0.6 μM. IC50 value: 0.6 uM [1]Target: alpha 2-adrenergic receptorin vitro: Yohimbine inhibits alpha2-receptor antagonist with Ki of 1.05 nM, 1.19 nM, and 1.19 nM for α2A, α2B, α2C, respectively. Yohimbine also inhibits 5-HT1B with Ki of 19.9 nM. Yohimbine acts to block the lowering of cAMP by alpha-2 adrenoceptor agonists. yohimbine actually causes a pronounced lowering of tyrosinase activity. [3]in vivo: Yohimbine is an antagonist at alpha2-noradrenaline receptors with putative panicogenic effects in human subjects, was administered to Swiss-Webster mice at doses of 0.5, 1.0, and 2.0 mg/kg. Yohimbine potentiates active defensive responses to threatening stimuli in Swiss-Webster mice.[2] |
|---|---|
| Related Catalog | |
| References |
| Density | 1.3±0.1 g/cm3 |
|---|---|
| Boiling Point | 543.0±50.0 °C at 760 mmHg |
| Melting Point | 231-233 °C(lit.) |
| Molecular Formula | C21H26N2O3 |
| Molecular Weight | 354.443 |
| Flash Point | 282.2±30.1 °C |
| Exact Mass | 354.194336 |
| PSA | 65.56000 |
| LogP | 2.20 |
| Vapour Pressure | 0.0±1.5 mmHg at 25°C |
| Index of Refraction | 1.661 |
| Storage condition | Store at RT |
CHEMICAL IDENTIFICATION
HEALTH HAZARD DATAACUTE TOXICITY DATA
|
| Hazard Codes | T |
|---|---|
| Risk Phrases | R23/24/25 |
| Safety Phrases | S27-S36/37/39-S45 |
| RIDADR | 1544 |
| RTECS | ZG1000000 |
| Packaging Group | II |
| Hazard Class | 6.1(a) |
| Precursor 6 | |
|---|---|
| DownStream 7 | |