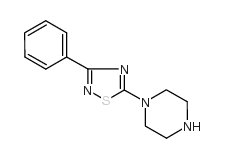
681136-29-8
| Name | N-phenyl-4-(3-phenyl-1,2,4-thiadiazol-5-yl)piperazine-1-carboxamide |
|---|---|
| Synonyms |
N1-phenyl-4-(3-phenyl-1,2,4-thiadiazol-5-yl)piperazine-1-carboxamide
N-Phenyl-4-(3-phenyl-1,2,4-thiadiazol-5-yl)piperazine-1-carboxamide N-Phenyl-4-(3-phenyl-1,2,4-thiadiazol-5-yl)-1-piperazinecarboxamide |
| Description | JNJ-1661010 (Takeda-25) a potent and selective fatty acid amide hydrolase (FAAH) inhibitor with IC50s of 34 and 33 nM for rat FAAH and human FAAH, respectively. JNJ-1661010 can cross the blood-brain barrier and used as broad-spectrum analgesics[1][2]. |
|---|---|
| Related Catalog | |
| Target |
IC50: 34 nM (rat FAAH) and 33 nM (human FAAH)[1] |
| In Vivo | JNJ-1661010 (Takeda-25; i.p.; 50 mg/kg) diminishes thermal hyperalgesia in the inflammatory rat carrageenan paw model[2]. JNJ-1661010 (i.p.; 10 mg/kg) has a T1/2 of 35 mins, a CL of 0.032 mL/min/kg, and a Cmax of 1.58 μM for rats[1]. |
| References |
| Density | 1.3±0.1 g/cm3 |
|---|---|
| Melting Point | 240.48 °C |
| Molecular Formula | C19H19N5OS |
| Molecular Weight | 365.452 |
| Exact Mass | 365.131042 |
| PSA | 89.60000 |
| LogP | 2.57 |
| Index of Refraction | 1.679 |
| Storage condition | Store at +4°C |
| Water Solubility | DMSO: ≥28mg/mL |
|
~82% 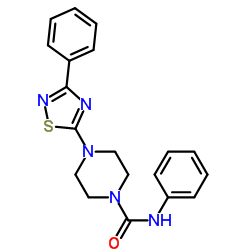
681136-29-8 |
| Literature: Takeda Pharmaceutical Company Limited Patent: EP1813606 A1, 2007 ; Location in patent: Page/Page column 32-33 ; |
|
~% 
681136-29-8 |
| Literature: Apodaca, Richard; Breitenbucher, J. Guy; Pattabiraman, Kanaka; Seierstad, Mark; Xiao, Wei Patent: US2007/4741 A1, 2007 ; Location in patent: Page/Page column 11; 14 ; |
| Precursor 3 | |
|---|---|
| DownStream 0 | |