480-23-9
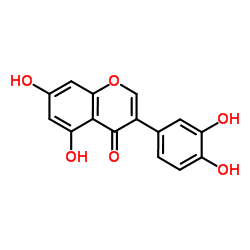
| Name | orobol |
|---|---|
| Synonyms |
3-(3,4-Dihydroxyphenyl)-5,7-dihydroxy-4H-chromen-4-one
5,7,3',4'-Tetrahydroxyisoflavone 3'-hydroxygenistein Orobol Norsantal 3',4',5,7-tetrahydroxyisoflavone Santol 3-(3,4-dihydroxyphenyl)-5,7-dihydroxychromen-4-one Isoluteolin |
| Description | Orobol is one of the major soy isoflavones and has various pharmacological activities, including anti-skin-aging and anti-obesity effects. Orobol inhibits CK1ε, VEGFR2, MAP4K5, MNK1, MUSK, TOPK, and TNIK (IC50=1.24-4.45 μM). Orobol also inhibits PI3K isoforms (IC50=3.46-5.27 μM for PI3K α/β/γ/K/δ)[1][2]. |
|---|---|
| Related Catalog | |
| In Vitro | Orobol binds to CK1ε in an ATP-competitive manner and exerts anti-obesity effects by targeting casein kinase 1 epsilon[2]. Orobol (5-20 μM) effectively suppresses MDI (isobutylmethylxanthine, dexamethasone and insulin (MDI))-induced phosphorylation of 4E-BP1[2]. |
| In Vivo | Orobol attenuates high fat diet-induced weight gain and lipid accumulation without affecting food intake in C57BL/6J mice[2]. Animal Model: HFD-induced obesity in C57BL/6J mice[2] Dosage: 10 mg/kg Administration: Intragastrically; daily for 23 weeks Result: Significantly reduced body weight by 17.3% compared to the HFD group. |
| References |
| Density | 1.7±0.1 g/cm3 |
|---|---|
| Boiling Point | 616.1±55.0 °C at 760 mmHg |
| Molecular Formula | C15H10O6 |
| Molecular Weight | 286.236 |
| Flash Point | 239.5±25.0 °C |
| Exact Mass | 286.047729 |
| PSA | 111.13000 |
| LogP | 2.76 |
| Vapour Pressure | 0.0±1.8 mmHg at 25°C |
| Index of Refraction | 1.768 |
CHEMICAL IDENTIFICATION
HEALTH HAZARD DATAACUTE TOXICITY DATA
|
| Hazard Codes | Xi |
|---|
|
~% 
480-23-9 |
| Literature: Chemler, Joseph A.; Lim, Chin Giaw; Daiss, John L.; Koffas, Mattheos A.G. Chemistry and Biology, 2010 , vol. 17, # 4 p. 392 - 401 |
|
~% 
480-23-9 |
| Literature: Narasimhachari; Seshadri Proceedings - Indian Academy of Sciences, Section A, 1950 , # 32 p. 342,345 |
|
~% 
480-23-9 |
| Literature: Anhut, Siegbert; Zinsmeister, H. Dietmar; Mues, Ruediger; Barz, Wolfgang; Mackenbrock, Klaus; et al. Phytochemistry (Elsevier), 1984 , vol. 23, # 5 p. 1073 - 1076 |
|
~% 
480-23-9 |
| Literature: Roberts-Kirchhoff, Elizabeth S.; Crowley, Jan R.; Hollenberg, Paul F.; Kim, Hyesook Chemical Research in Toxicology, 1999 , vol. 12, # 7 p. 610 - 616 |
|
~% 
480-23-9 |
| Literature: Heerden, Fanie R. van; Brandt, E. Vincent; Roux, David G. Journal of the Chemical Society, Perkin Transactions 1: Organic and Bio-Organic Chemistry (1972-1999), 1980 , p. 2463 - 2469 |
|
~% 
480-23-9 |
| Literature: Heerden, Fanie R. van; Brandt, E. Vincent; Roux, David G. Journal of the Chemical Society, Perkin Transactions 1: Organic and Bio-Organic Chemistry (1972-1999), 1980 , p. 2463 - 2469 |
| Precursor 4 | |
|---|---|
| DownStream 0 | |