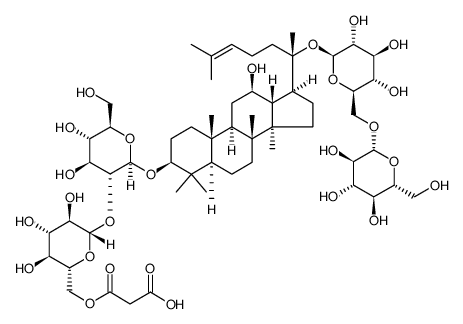
41753-43-9
| Name | ginsenoside Rb1 |
|---|---|
| Synonyms |
gypenosideiii
(3β,12β)-20-{[6-O-(β-D-Glucopyranosyl)-β-D-glucopyranosyl]oxy}-12-hydroxydammar-24-en-3-yl 2-O-β-D-glucopyranosyl-β-D-glucopyranoside EINECS 255-532-8 Ginsenoside-Rb1 arasaponine1 gynosaponinc ginseng Rb1 Gypenoside I ginsenoside Rb1 Panaxoside Rb1 GYPENOSIDE panaxsaponine GinsenosideRb1 Ginsenoside-Rb1 from Panax ginseng (Korean ginseng) root MFCD00133367 SANCHINOSIDE E1 ginsinoside Rb1 |
| Description | Ginsenoside Rb1, a main constituent of the root of Panax ginseng, inhibits Na+, K+-ATPase activity with an IC50 of 6.3±1.0 μM. Ginsenoside also inhibits IRAK-1 activation and phosphorylation of NF-κB p65 . |
|---|---|
| Related Catalog | |
| Target |
Na+, K+-ATPase:6.3 μM (IC50) IRAK-1 p65 Autophagy Mitophagy |
| In Vitro | Rat brain microsomal Na+, K+-ATPase activity is inhibited significantly and rapidly by Ginsenoside Rb1. The IC50 of Ginsenoside Rb1 for Na+,K+-ATPase is 6.3±1.0 μM. The inhibition is enhanced with increasing the concentration of Ginsenoside Rb1 or decreasing that of Na+ and K+. Kinetic analysis reveals that Ginsenoside is an uncompetitive inhibitor with respect to ATP[1].Ginsenoside Rb1 significantly inhibits the activation of interleukin-1 receptor-associated kinase-1 (IRAK-1), IKK-β, NF-κB, and MAP kinases (ERK, JNK, and p-38); however, interaction between LPS and Toll-like receptor-4, IRAK-4 activation and IRAK-2 activation are unaffected[2]. Ginsenoside Rb1 is an ingredient of a Chinese medicine Panax ginseng. Ginsenoside Rb1 is a major bioactive compound in the regulating pregnane X receptor (PXR)/NF-κB signaling. Ginsenoside Rb1 is the compound with potent anti-inflammatory activity in ginseng saponin extract (GSE). The concentration for Ginsenoside Rb1 (10 μM) is optimized from a preliminary study to ensure sufficient anti-inflammatory activity and without apparent cytotoxicity. Ginsenoside Rb1 significantly reduces TNF-α-induced upregulation of IL-1β and iNOS mRNA levels, and restores the mRNA levels of PXR and CYP3A4 in LS174T cells. TNF-α causes a significant reduction in PXR protein levels and increase in the ratio of phosphorylated to total NF-κB p65, both of which are significantly abrogated by Ginsenoside Rb1[3]. |
| In Vivo | Ginsenoside Rb1 at the both doses of 30 mg/kg and 60 mg/kg significantly attenuates the histological lung injury. Ginsenoside Rb1 at the dose of 30 mg/kg and 60 mg/kg both significantly attenuates the histological intestine injury[4]. Ginsenoside Rb1 (Rb1), an ingredient of a Chinese medicine Panax ginseng, has beneficial effects on mesentery microvascular hyperpermeability induced by Lipopolysaccharide (LPS) and the underlying mechanisms. In some rats, Ginsenoside Rb1 (5 mg/kg per hour) is administrated through the left jugular vein 30 min after LPS infusion. Ginsenoside Rb1 decreases caveolae number in endothelial cells of microvessels. Ginsenoside Rb1 ameliorates microvascular hyperpermeability after the onset of endotoxemia and improves intestinal edema through inhibiting caveolae formation and junction disruption, which is correlated to suppression of NF-κB and Src activation[5]. |
| Cell Assay | LS174T cells are seeded in cell imaging dish. After overnight incubation, cells are treated with GSE (100 μg/mL), Ginsenoside Rb1 (10 μM), or CK (10 μM) for 3 hours, followed by an additional incubation with or without TNF-α (20 ng/ml) for 6 hours. At the end of the incubation, cells are harvested and fixed with 4% paraformaldehyde solution at 20°C for 20 minutes. After washing in PBS, cells are permeabilized with 0.2% Triton X-100 in PBS at room temperature for 5 minutes. After incubation in blocking buffer containing 0.1% Triton X-100 and 5% bovine serum albumin, cells are incubated with rabbit NF-κB p65 antibody at 4°C overnight and then with Alexa Fluor 488-conjugated anti-rabbit IgG antibody at room temperature for 30 minutes in 1% bovine serum albumin in PBS. Fluorescence photographs are obtained using a Zeiss 710 confocal microscope[3]. |
| Animal Admin | Mice[4] Male C57BL/6 mice (9-12 weeks old; 17-22 g) are randomly allocated into eight groups (n=8 in each group): (1) Sham surgical preparation including isolation of the superior mesenteric artery (SMA) without occlusion is performed (Sham); (2) mice are subjected to II/R without treatment (II/R); (3) mice are subjected to II/R with treatment of normal saline 10 minutes before reperfusion (II/R+NS); (4), (5) mice are treated with 30 mg/kg (II/R+Rb1-30) or 60 mg/kg (II/R+Rb1-60) Ginsenoside Rb1, in which surgery is performed as in the II/R group with administration of the Ginsenoside Rb1 intraperitoneally 10 minutes before reperfusion; (6) mice are subjected to Sham surgery and treated with ATRA (ATRA+Sham), which is the inhibitor of Nrf2/ARE signaling pathway; (7) mice are subjected to II/R and treated with ATRA (ATRA+II/R); (8) mice are subjected to II/R and treated with ATRA and 60 mg/kg Ginsenoside Rb1 as group 5 (ATRA+II/R+Rb1-60). During the last two weeks before the operation, the mice in the group 6, 7, 8 receive ATRA i.p. daily at 10 mg/kg and fed on a vitamin A-deficient diet, and the mice in the other groups receive the equivalent volume of corn oil and fed on a control normal diet. Rats[5] Male Wistar rats weighing 200-250 g are used. The rats are randomly divided into four groups, 26 animals in each. After being anesthetized with urethane (2 g/kg body wt im), the left femoral vein and left jugular vein of the rat are cannulated. In the LPS group, LPS solution in saline is infused (5 mg/kg per hour) for 90 min via the left femoral vein. The vehicle, instead of LPS solution, is administrated in Sham and Ginsenoside Rb1 alone (Rb1, 5 mg/kg) groups. In the Rb1 posttreatment (LPS+Rb1) group, Ginsenoside Rb1 solution is infused continuously through the left jugular vein 30 min after LPS administration at the dose of 5 mg/kg. Ginsenoside Rb1 solution and the same volume of saline are infused in Ginsenoside Rb1 and Sham groups, respectively, without subsequent LPS administration. In a separate set of experiments, the rats are anesthetized with 2% penobarbital sodium (60 mg/kg body wt ip), and saline, LPS, and Ginsenoside Rb1. After recovery from anesthesia, the animals are allowed access to water and rodent chow, and survival rate is recorded over time until 4 days after LPS stimulation. |
| References |
| Density | 1.4±0.1 g/cm3 |
|---|---|
| Boiling Point | 1145.9±65.0 °C at 760 mmHg |
| Molecular Formula | C54H92O23 |
| Molecular Weight | 1109.295 |
| Flash Point | 646.8±34.3 °C |
| Exact Mass | 1108.602905 |
| PSA | 377.29000 |
| LogP | 2.90 |
| Vapour Pressure | 0.0±0.6 mmHg at 25°C |
| Index of Refraction | 1.626 |
| Storage condition | 2-8°C |
CHEMICAL IDENTIFICATION
HEALTH HAZARD DATAACUTE TOXICITY DATA
MUTATION DATA
|
| Symbol |

GHS07 |
|---|---|
| Signal Word | Warning |
| Hazard Statements | H302 + H312 + H332 |
| Precautionary Statements | P261-P280-P301 + P312 + P330 |
| Personal Protective Equipment | dust mask type N95 (US);Eyeshields;Gloves |
| Hazard Codes | Xi:Irritant; |
| Risk Phrases | R20/21/22 |
| Safety Phrases | S26-S36 |
| RIDADR | UN 1230 3/PG 2 |
| WGK Germany | 3 |
| RTECS | LZ5856000 |
|
~% 
41753-43-9 |
| Literature: Kitagawa; Taniyama; Yoshikawa; Ikenishi; Nakagawa Chemical and Pharmaceutical Bulletin, 1989 , vol. 37, # 11 p. 2961 - 2970 |
|
~% 
41753-43-9 |
| Literature: Kitagawa; Taniyama; Yoshikawa; Ikenishi; Nakagawa Chemical and Pharmaceutical Bulletin, 1989 , vol. 37, # 11 p. 2961 - 2970 |
| Precursor 1 | |
|---|---|
| DownStream 1 | |