121207-31-6
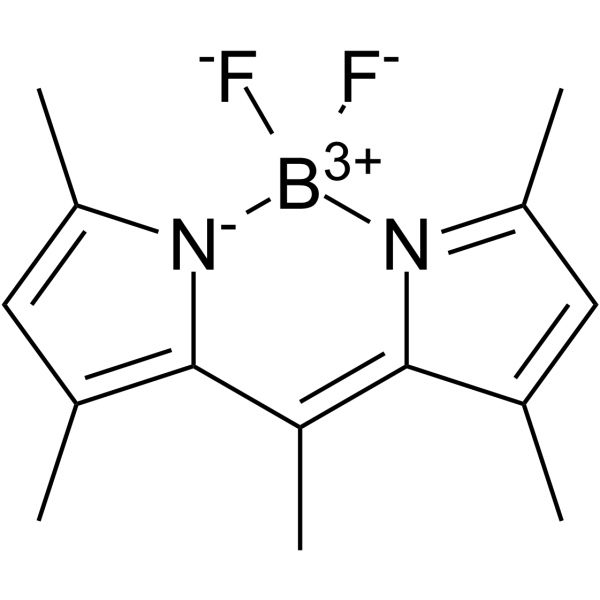
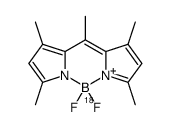
| Name | 4,4-Difluoro-1,3,5,7,8-pentamethyl-4-bora-3a,4a-diaza-s-indacene |
|---|---|
| Synonyms |
1,3,5,7,8-pentamethyl-4,4-difluoro-4-bora-3a,4a-diaza-s-indacene
4,4-difluoro-1,3,5,7,8-pentamethyl-4-bora-3a,4a,diaza-s-indacene Difluoro{2-[1-(3,5-dimethyl-2H-pyrrol-2-ylidene-N)ethyl]-3,5-dimethyl-1H-pyrrolato-N}boron 4,4-difluoro-1,3,5,7,8-pentamethyl-BODIPY Pyrromethene 546 4,4-difluoro-1,3,4,5,7-quantmethyl-4-bora-3a,4a-diaza-s-indacene BODIPY 493/503 |
| Description | BODIPY 493/503 (Pyrromethene 546) is a fluorescent neutral lipid dye (Ex/Em: 493/503 nm). BODIPY 493/503 can facilitate quantification of neutral lipid content by flow cytometry and observation of lipid droplets (LDs) by microscopy[1]. |
|---|---|
| Related Catalog | |
| In Vitro | Guidelines (Following is our recommended protocol. This protocol only provides a guideline, and should be modified according to your specific needs). A. BODIY staining for flow cytometry 1. Grow cells under culture conditions relevant for the study. 2. At the time-point of interest, prepare 2 μM BODIPY staining solution in PBS. The volume of staining solution required for each sample corresponds to the volume of media used for culturing cells. 3. Wash cells with a quick rinse using 3 mL PBS to remove media/serum. 4. Incubate on BODIPY staining solution in the dark for 15 min at 37°C. Include an unstained control for flow cytometry. (Note: From this point, protect samples from light as much as possible) 5. Wash cells with a quick rinse using 3 mL PBS to remove staining solution. 6. Trypsinize cells to generate a single cell suspension. 7. Add 5 mL of PBS and transfer cell suspension to a 15 mL conical tube. 8. Pellet cells at 250×g, 5 min, 4 °C. 9. Aspirate supernatant, wash the cell pellet with a quick rinse using 3 mL PBS, and pellet cells at 250×g, 5 min, 4°C. 10. Carefully aspirate the supernatant and resuspend cells in 300 μL 1×flow cytometry buffer. 11. Pass cell suspension through a 35 μM filter into a FACS tube. 12. Perform flow cytometry. Obtain a minimum of 10,000 events per condition. 13. The investigator can analyze data as mean fluorescence or display the data as a histogram[1]. B. BODIY staining for microscopy 1. Autoclave coverslips in a glass bottle. 2. In the tissue culture hood, place coverslips into 35 mm cell culture dishes. 3. Prepare 2 mg/mL collagen solution in PBS. 4. Treat the coverslips with collagen to promote cell adherence. Add 3 mL collagen solution to culture dishes and incubate at 37°C for 30 min. Note: Use forceps to ensure that coverslips are flush with the bottom of the culture dish, eliminating any air bubbles that may be under the cover slips. 5. Aspirate the collagen solution. 6. Wash with PBS. 7. Add PBS to culture dishes and place under UV light in the culture hood to sterilize. 8. Plate cells into culture dishes containing the coverslips. The optimal cell number should be determined to achieve confluence of 30-50% at the time of staining to permit proper imaging. 9. Incubate under the culture conditions relevant to your experiment. 10. At the time-point of interest, prepare 2 μM BODIPY staining solution in PBS. 11. Wash cells with 3 mL PBS. 12. Incubate on 3 mL staining solution for 15 min at 37 °C. (Note: From this point, protect samples from light as much as possible) 13. Wash twice in 3 mL PBS. 14. Fix cells in 3 mL 4% PFA for 30 min at room temperature. 15. Remove 4% PFA. 16. Wash samples 3×5 min in PBS. 17. Use forceps to mount cover slips onto glass slides. 18. Allow the mounting solution to cure overnight at room temperature. 19. Slides can be stored at 4°C or imaged immediately[1]. |
| References |
| Melting Point | 257 °C |
|---|---|
| Molecular Formula | C14H17BF2N2 |
| Molecular Weight | 262.10600 |
| Exact Mass | 262.14500 |
| PSA | 13.32000 |
| LogP | 2.82650 |
| Symbol |

GHS07 |
|---|---|
| Signal Word | Warning |
| Hazard Statements | H315-H319-H335 |
| Precautionary Statements | P261-P305 + P351 + P338 |
| RIDADR | NONH for all modes of transport |