89565-68-4
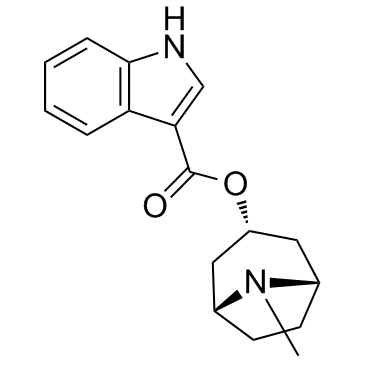
| Name | tropisetron |
|---|---|
| Synonyms |
1aH,5aH-Tropan-3a-yl indole-3-carboxylate
TROPICACID ICF 205-930 1H-Indole-3-carboxylic acid, (3-endo)-8-methyl-8-azabicyclo[3.2.1]oct-3-yl ester SS-TROPISETRON 3-Tropanylindole-3-carboxylate 3a-Tropanyl-1H-indole-3-carboxylic acid ester UNII-6I819NIK1W Indole-3-carbonyl chloride MFCD00864399 Tropisetron endo-1H-Indole-3-carboxylic acid 8-methyl-8-azabicyclo[3.2.1]oct-3-yl ester icf205-930 (3-endo)-8-Methyl-8-azabicyclo[3.2.1]oct-3-yl 1H-indole-3-carboxylate ICS 205-930 TROPISHTRON HYDROCHLORIDE |
| Description | Tropisetron(SDZ-ICS 930) is a selective 5-HT3 receptor antagonist and α7-nicotinic receptor agonist with an IC50 of 70.1 ± 0.9 nM for 5-HT3 receptor. IC50 value: 70.1 ± 0.9 nM [1]Target: 5-HT3 receptorin vitro: Tropisetron specifically inhibited both IL-2 gene transcription and IL-2 synthesis in stimulated T cells. tropisetron inhibited both the binding to DNA and the transcriptional activity of NFAT and AP-1. We also observed that tropisetron is a potent inhibitor of PMA plus ionomycin-induced NF-(kappa)B activation but in contrast TNF(alpha)-mediated NF-(kappa)B activation was not affected by this antagonist [2]. Tropisetron prevents the phosphorylation and thus activation of the p38 MAPK, which is involved in post-transcriptional regulation of various cytokines [3].in vivo: Two different doses of tropisetron (5 and 10 mg/kg) or vehicle were administered intraperitoneally 30 min before pMCAO. Neurological deficit scores, mortality rate and infarct volume were determined 24 h after permanent focal cerebral ischemia [4]. |
|---|---|
| Related Catalog | |
| References |
| Density | 1.3±0.1 g/cm3 |
|---|---|
| Boiling Point | 448.5±35.0 °C at 760 mmHg |
| Melting Point | 201-202 °C |
| Molecular Formula | C17H20N2O2 |
| Molecular Weight | 284.35 |
| Flash Point | 225.0±25.9 °C |
| PSA | 83.80000 |
| LogP | 3.55 |
| Vapour Pressure | 0.0±1.1 mmHg at 25°C |
| Index of Refraction | 1.644 |
| Storage condition | 2-8°C |
| Water Solubility | H2O: soluble |
| Hazard Codes | Xi |
|---|---|
| WGK Germany | 3 |
|
~% 
89565-68-4 |
| Literature: European Journal of Medicinal Chemistry, , vol. 28, # 11 p. 869 - 880 |
| Precursor 2 | |
|---|---|
| DownStream 0 | |