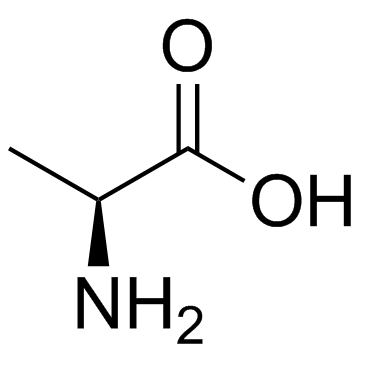
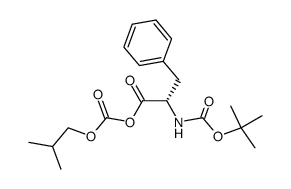
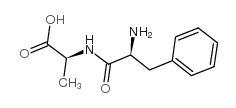
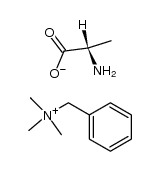
55677-48-0
| Name | (2S)-2-[[(2S)-2-[(2-methylpropan-2-yl)oxycarbonylamino]-3-phenylpropanoyl]amino]propanoic acid |
|---|---|
| Synonyms |
t-Butyloxycarbonylphenylalanylalanine
Boc-phenylalanyl-alanine t-butyloxycarbonyl-L-phenylalanyl-L-alanine Boc-phe-ala BOC-L-PHENYLALANYL ALANINE N-tert-butyloxycarbonyl-L-phenylalanyl-L-alanine Boc-Phe-Ala-OH N-Boc-PheAla |
| Description | (tert-Butoxycarbonyl)-L-phenylalanyl-L-alanine is an alanine derivative[1]. |
|---|---|
| Related Catalog | |
| In Vitro | Amino acids and amino acid derivatives have been commercially used as ergogenic supplements. They influence the secretion of anabolic hormones, supply of fuel during exercise, mental performance during stress related tasks and prevent exercise induced muscle damage. They are recognized to be beneficial as ergogenic dietary substances[1]. |
| References |
| Density | 1.182g/cm3 |
|---|---|
| Boiling Point | 587.8ºC at 760mmHg |
| Melting Point | 90-93℃ |
| Molecular Formula | C17H24N2O5 |
| Molecular Weight | 336.38300 |
| Flash Point | 309.3ºC |
| Exact Mass | 336.16900 |
| PSA | 104.73000 |
| LogP | 2.49350 |
| Index of Refraction | 1.53 |
| Storage condition | 2-8℃ |
|
~78% 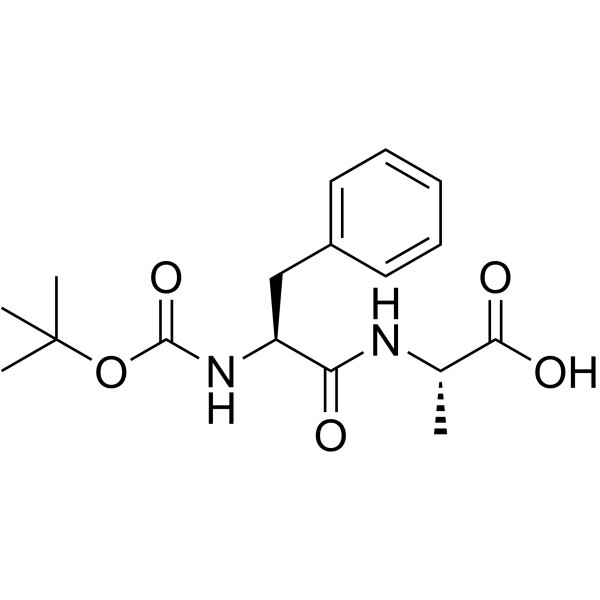
55677-48-0 |
| Literature: Fustero, Santos; Sancho, Amador Garcia; Chiva, Gema; Sanz-Cervera, Juan F.; Del Pozo, Carlos; Acena, Jose Luis Journal of Organic Chemistry, 2006 , vol. 71, # 8 p. 3299 - 3302 |
|
~95% 
55677-48-0 |
| Literature: Katritzky, Alan R.; Suzuki, Kazuyuki; Singh, Sandeep K. Synthesis, 2004 , # 16 p. 2645 - 2652 |
|
~83% 
55677-48-0 |
| Literature: Popovic, Stanimir; Bieraeugel, Hans; Detz, Remko J.; Kluwer, Alexander M.; Koole, Jelmer A. A.; Streefkerk, Dieuwertje E.; Hiemstra, Henk; Van Maarseveen, Jan H. Chemistry - A European Journal, 2013 , vol. 19, # 50 p. 16934 - 16937 |
|
~86% 
55677-48-0 |
| Literature: Martinez, Jean; Laur, Janine; Castro, Bertrand Tetrahedron, 1985 , vol. 41, # 4 p. 739 - 744 |
|
~% 
55677-48-0 |
| Literature: Gewehr, Markus; Kunz, Horst Synthesis, 1997 , # 12 p. 1499 - 1510 |
|
~% 
55677-48-0 |
| Literature: Verardo; Gorassini Journal of Peptide Science, 2013 , vol. 19, # 5 p. 315 - 324 |
|
~% 
55677-48-0 |
| Literature: Zajdlik, Adam; Wang, Zezhou; Hickey, Jennifer L.; Aman, Ahmed; Schimmer, Aaron D.; Yudin, Andrei K. Angewandte Chemie - International Edition, 2013 , vol. 52, # 32 p. 8411 - 8415 Angew. Chem., 2013 , vol. 125, # 32 p. 8569 - 8573 |
|
~% 
55677-48-0 |
| Literature: Chen, Shui-Tein; Chang, Chung-Ho; Wang, Kung-Tsung Journal of Chemical Research, Miniprint, 1991 , # 8 p. 1967 - 1975 |
| Precursor 8 | |
|---|---|
| DownStream 0 | |