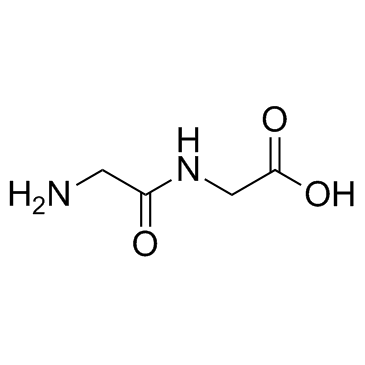
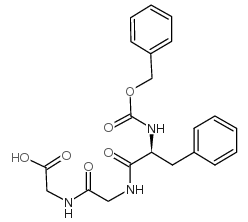
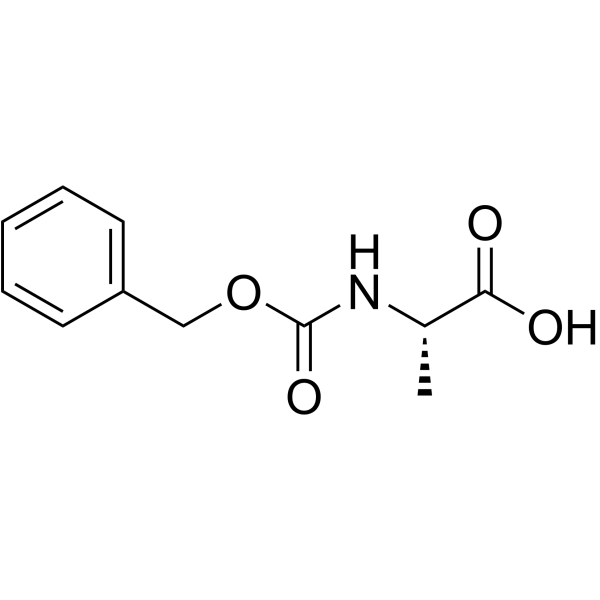
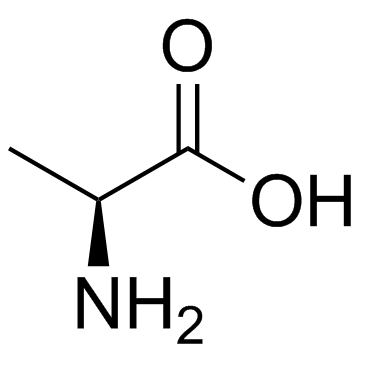
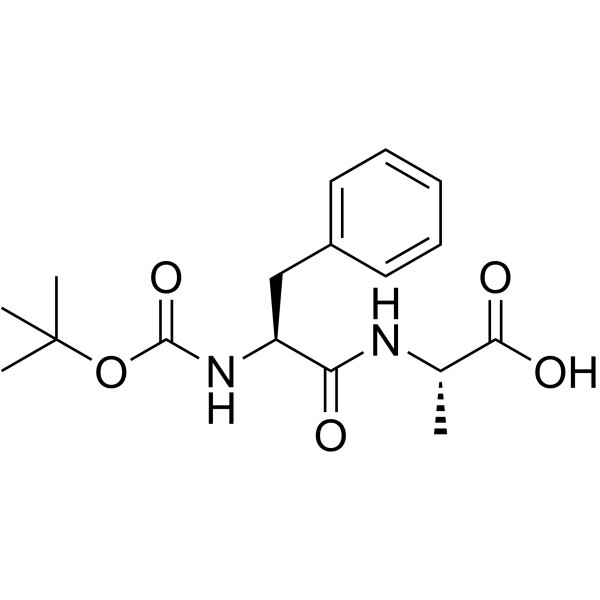
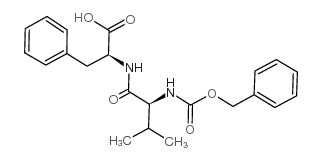
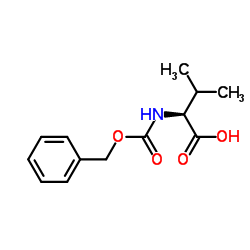
|
~98% |
|
~% |
|
~10% |
|
~% |
|
~% |
|
~95% |
|
~96% |
|
~95% |
|
~% |

![(S)-Benzyl 1-(1H-benzo[d][1,2,3]triazol-1-yl)-1-oxo-3-phenylpropan-2-ylcarbamate Structure](https://image.chemsrc.com/caspic/275/769922-77-2.png)