1346169-63-8
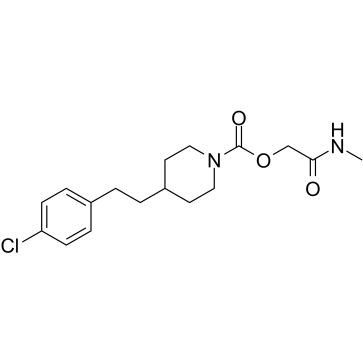
| Name | 2-(Methylamino)-2-oxoethyl 4-[2-(4-chlorophenyl)ethyl]-1-piperidinecarboxylate |
|---|---|
| Synonyms |
2-(Methylamino)-2-oxoethyl 4-[2-(4-chlorophenyl)ethyl]-1-piperidinecarboxylate
1-Piperidinecarboxylic acid, 4-[2-(4-chlorophenyl)ethyl]-, 2-(methylamino)-2-oxoethyl ester |
| Description | SA57 is a potent, selective FAAH inhibitor with IC50s of 3.2 nM and 1.9 nM for mouse and human FAAH. SA57 also inhibits the 2-arachidonoylglycerol hydrolases MAGL (IC50s of 410 nM and 1.4 μM for mouse and human MAGL) and mouse α/β-hydrolase domain-containing protein 6 (mABHD6; IC50 of 850 nM), but not other brain serine hydrolases[1][2]. |
|---|---|
| Related Catalog | |
| Target |
IC50: 3.2 nM (Mouse FAAH) and 1.9 nM (Human FAAH); 410 nM (Mouse MAGL) and 1.4 μM (Human MAGL); 850 nM (Mouse ABHD6)[1] |
| In Vitro | O-Aryl carbamate and N-aryl urea inhibitors have been shown to irreversibly inhibit FAAH by carbamylation of the enzyme’s serine nucleophile. SA57 exhibits clear time-dependent inhibition of FAAH and MAGL, suggesting a covalent mechanism of inactivation, presumably through carbamylation of the active site serine nucleophiles of these enzymes[1]. |
| In Vivo | SA57 (0.01-12.5 mg/kg; intraperitoneal injection; for 2 hours; C57Bl/6 mice) treatment shows distinct dose-responsive activity against brain serine hydrolases (FAAH, MAGL and ABHD6) in vivo[1]. Animal Model: C57Bl/6 mice[1] Dosage: 0.01 mg/kg, 0.05 mg/kg, 0.25 mg/kg, 1.25 mg/kg, 6.25 mg/kg, 12.5 mg/kg Administration: Intraperitoneal injection; for 2 hours Result: Showed distinct dose-responsive activity against brain serine hydrolases. Inhibited FAAH, MAGL and ABHD6 in vivo. |
| References |
| Density | 1.2±0.1 g/cm3 |
|---|---|
| Boiling Point | 512.1±30.0 °C at 760 mmHg |
| Molecular Formula | C17H23ClN2O3 |
| Molecular Weight | 338.829 |
| Flash Point | 263.5±24.6 °C |
| Exact Mass | 338.139709 |
| LogP | 2.71 |
| Vapour Pressure | 0.0±1.3 mmHg at 25°C |
| Index of Refraction | 1.540 |
| Hazard Codes | Xi |
|---|