491-54-3
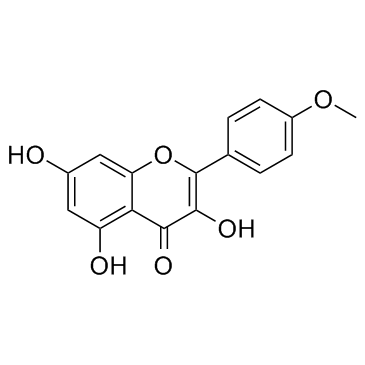
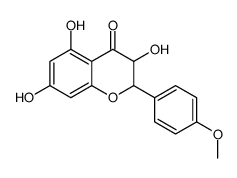
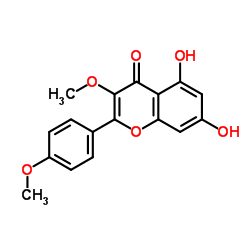
| Name | kaempferide |
|---|---|
| Synonyms |
4'-O-Methylkaempferol
Kaempferol 4'-methyl ether 3,5,7-trihydroxy-2-(4-methoxyphenyl)chromen-4-one 3,5,7-Trihydroxy-2-(4-methoxyphenyl)-4H-chromen-4-one 3,5,7-trihydroxy-4'-methoxyflavone Kaempferid Kaempferide 3 5 7-trihydroxy-4'-methoxyflavone 4'-Methoxy-3,5,7-trihydroxyflavone 3,5,7-trihydroxy-4′-methoxyflavone 4'-Methylkaempferol |
| Description | Kaempferide is an O-methylated flavonol, a type of chemical compound. It can be found in Kaempferia galanga (aromatic ginger). The enzyme kaempferol 4'-O-methyltransferase uses S-adenosyl-L-methionine and kaempferol to produce S-adenosyl-L-homocysteine and kaempferide. P-glycoproteins. |
|---|---|
| Related Catalog | |
| References |
| Density | 1.5±0.1 g/cm3 |
|---|---|
| Boiling Point | 543.8±50.0 °C at 760 mmHg |
| Melting Point | 156-157ºC(lit.) |
| Molecular Formula | C16H12O6 |
| Molecular Weight | 300.263 |
| Flash Point | 207.1±23.6 °C |
| Exact Mass | 300.063385 |
| PSA | 100.13000 |
| LogP | 2.74 |
| Vapour Pressure | 0.0±1.5 mmHg at 25°C |
| Index of Refraction | 1.710 |
| Storage condition | 2-8°C |
CHEMICAL IDENTIFICATION
HEALTH HAZARD DATAACUTE TOXICITY DATAMUTATION DATA
|
| Hazard Codes | Xi |
|---|---|
| Risk Phrases | R36/37/38 |
| Safety Phrases | S26-S36 |
| RIDADR | NONH for all modes of transport |
| WGK Germany | 2 |
| RTECS | KD4170000 |
| HS Code | 29329990 |
| Precursor 9 | |
|---|---|
| DownStream 5 | |








![[3,5-diacetyloxy-2-(4-methoxyphenyl)-4-oxochromen-7-yl] acetate structure](https://image.chemsrc.com/caspic/355/38681-32-2.png)