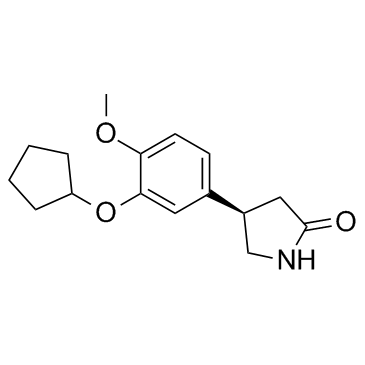
61413-54-5
| Name | rolipram |
|---|---|
| Synonyms |
4-[3-(Cyclopentyloxy)-4-methoxyphenyl]pyrrolidin-2-one
(R,S)-Rolipram 4-(3-cyclopentyloxy-4-methoxy-phenyl)-pyrrolidin-2-one (±)-Rolipram Adeo [3H]-Rolipram Rolipram 4-(3-cyclopentyloxy-4-methoxyphenyl)pyrrolidin-2-one 4-[3-(Cyclopentyloxy)-4-methoxyphenyl]-2-pyrrolidinone EINECS 262-771-1 Rolipramum [Latin] T5MVTJ DR DO1 CO- AL6TJ MFCD00270906 |
| Description | Rolipram is a selective phosphodiesterases PDE4 inhibitor with IC50s of 3 nM, 130 nM and 240 nM for PDE4A, PDE4B, and PDE4D, respectively. |
|---|---|
| Related Catalog | |
| Target |
IC50: 3 nM (PDE4A), 130 nM (PDE4B), 240 nM (PDE4D)[1] |
| In Vitro | The PDE4 selective inhibitor, Rolipram, inhibits immunopurified PDE4B and PDE4D activities similarly, with IC50s of approx. 130 nM and 240 nM respectively. In contrast, Rolipram inhibits immunopurified PDE4A activity with a dramatically lower IC50 of around 3 nM. Rolipram increases phosphorylation of cAMP-response-element-binding protein (CREB) in U937 cells in a dose-dependent fashion, which implies the presence of both high affinity (IC50 approx. 1 nM) and low affinity (IC50 approx. 120 nM) components. Rolipram dose-dependently inhibits the IFN-gamma-stimulated phosphorylation of p38 MAPK in a simple monotonic fashion with an IC50 of approx. 290 nM[1]. Rolipram is a selective PDE4 inhibitor that inhibits all PDE4 isoforms A, B, C and D. Rolipram inhibits LPS-induced TNF production in a dose-dependent manner (IC50 25.9 nM), and maximal/submaximal inhibition is observed with 2 μM drug concentration in J774 cells[2]. |
| In Vivo | TNF mRNA and protein expression is induced by LPS in peritoneal macrophages (PM) from WT mice, and that is clearly (by 74 and 63% for TNF mRNA and TNF protein, respectively) inhibited by Rolipram. LPS-induced TNF production is enhanced in PM from MKP-1(-/-) mice as compared to that in PM from WT mice, which is in line with the published results. Interestingly, the inhibition of TNF mRNA and protein expression by Rolipram is markedly attenuated in PM from MKP-1(-/-) mice and does not reach statistical significance[2]. Repeated administration of Rolipram (1.25 mg/kg, i.p.) reduces the number of escape failures in learned helplessness rats[3]. |
| Cell Assay | J774 murine macrophages (ATCC) are cultured at 37°C in 5% CO2 atmosphere in DMEM supplemented with glutamax-1 containing 10% heat-inactivated FBS, 100 U/mL penicillin, 100 μg/mL streptomycin and 250 ng/mL amphotericin B. For experiments, cells are seeded on 24-well plates at a density of 2×105 cells per well. Cell monolayers are grown for 72 h before the experiments are started. Rolipram, IBMX and BIRB 796 are dissolved in DMSO, and 8-Br-cAMP in HBSS. LPS (10 ng/mL) or the compounds of interest at concentrations indicated or the solvent (DMSO, 0.1% v/v) are added to the cells in fresh culture medium containing 10% FBS and the supplements. Cells are further incubated for the time indicated. The effect of LPS and the tested chemicals on cell viability is evaluated by Cell Proliferation Kit II (XTT)[2]. |
| Animal Admin | Mice[2] Inbred C57BL/6 MKP-1(-/-) mice are used. C57BL/6 mice (20-25 g) are divided into groups of six mice and treated with 200 μL of PBS or Rolipram (100 mg/kg in PBS) by an i.p. injection 2 h before applying carrageenan. Before the administration of carrageenan, the mice are anaesthetized by i.p. injection of 0.5 mg/kg of medetomidine and 75 mg/kg of ketamine. The mice receive a 30 μL i.d. injection of carrageenan (1.5%, dissolved in normal saline) in one hind paw. The contralateral paw receive 30 μL of saline and it is used as a control. Paw volume is measured before and 3 h after the carrageenan injection with a plethysmometer. Oedema is expressed as a change in paw volume over time. |
| References |
| Density | 1.2±0.1 g/cm3 |
|---|---|
| Boiling Point | 472.7±45.0 °C at 760 mmHg |
| Melting Point | 127-133ºC |
| Molecular Formula | C16H21NO3 |
| Molecular Weight | 275.343 |
| Flash Point | 239.7±28.7 °C |
| Exact Mass | 275.152130 |
| PSA | 47.56000 |
| LogP | 1.43 |
| Vapour Pressure | 0.0±1.2 mmHg at 25°C |
| Index of Refraction | 1.552 |
| Storage condition | 0-6°C |
| Water Solubility | H2O: 0.2 mg/mL |
CHEMICAL IDENTIFICATION
HEALTH HAZARD DATAACUTE TOXICITY DATA
|
| Personal Protective Equipment | dust mask type N95 (US);Eyeshields;Gloves |
|---|---|
| Hazard Codes | Xi |
| Risk Phrases | R36/37/38 |
| Safety Phrases | 26-36 |
| RIDADR | UN 3249 |
| WGK Germany | 3 |
| RTECS | UY5749237 |
| Packaging Group | III |
| Hazard Class | 6.1(b) |
| HS Code | 2933790090 |
| Precursor 6 | |
|---|---|
| DownStream 2 | |
| HS Code | 2933790090 |
|---|---|
| Summary | 2933790090. other lactams. VAT:17.0%. Tax rebate rate:9.0%. . MFN tariff:9.0%. General tariff:20.0% |



![[2-(3-cyclopentyloxy-4-methoxyphenyl)ethyl]-(2,4,6-trimethylbenzyl)amine structure](https://image.chemsrc.com/caspic/360/569342-89-8.png)