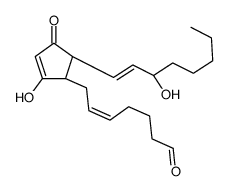
CHEMICAL IDENTIFICATION
-
RTECS NUMBER :
-
UK8000000
-
CHEMICAL NAME :
-
Prosta-5,13-dien-1-oic acid, (5Z,11-alpha,13E,15S)-11,15-dihydroxy-9-oxo-
-
CAS REGISTRY NUMBER :
-
363-24-6
-
LAST UPDATED :
-
199603
-
DATA ITEMS CITED :
-
72
-
MOLECULAR FORMULA :
-
C20-H32-O5
-
MOLECULAR WEIGHT :
-
352.52
-
WISWESSER LINE NOTATION :
-
L5VTJ B2U4VQ C1U1YQ5 DQ
HEALTH HAZARD DATA
ACUTE TOXICITY DATA
-
TYPE OF TEST :
-
LD50 - Lethal dose, 50 percent kill
-
ROUTE OF EXPOSURE :
-
Oral
-
SPECIES OBSERVED :
-
Rodent - rat
-
DOSE/DURATION :
-
500 mg/kg
-
TOXIC EFFECTS :
-
Behavioral - somnolence (general depressed activity) Gastrointestinal - hypermotility, diarrhea Skin and Appendages - hair
-
TYPE OF TEST :
-
LD50 - Lethal dose, 50 percent kill
-
ROUTE OF EXPOSURE :
-
Subcutaneous
-
SPECIES OBSERVED :
-
Rodent - rat
-
DOSE/DURATION :
-
31600 ug/kg
-
TOXIC EFFECTS :
-
Gastrointestinal - hypermotility, diarrhea Skin and Appendages - dermatitis, other (after systemic exposure) Skin and Appendages - hair
-
TYPE OF TEST :
-
LD50 - Lethal dose, 50 percent kill
-
ROUTE OF EXPOSURE :
-
Intravenous
-
SPECIES OBSERVED :
-
Rodent - rat
-
DOSE/DURATION :
-
59500 ug/kg
-
TOXIC EFFECTS :
-
Behavioral - somnolence (general depressed activity) Gastrointestinal - hypermotility, diarrhea Skin and Appendages - hair
-
TYPE OF TEST :
-
LD50 - Lethal dose, 50 percent kill
-
ROUTE OF EXPOSURE :
-
Oral
-
SPECIES OBSERVED :
-
Rodent - mouse
-
DOSE/DURATION :
-
750 mg/kg
-
TOXIC EFFECTS :
-
Behavioral - somnolence (general depressed activity) Gastrointestinal - hypermotility, diarrhea Skin and Appendages - hair
-
TYPE OF TEST :
-
LD50 - Lethal dose, 50 percent kill
-
ROUTE OF EXPOSURE :
-
Subcutaneous
-
SPECIES OBSERVED :
-
Rodent - mouse
-
DOSE/DURATION :
-
19700 ug/kg
-
TOXIC EFFECTS :
-
Gastrointestinal - hypermotility, diarrhea Skin and Appendages - dermatitis, other (after systemic exposure) Skin and Appendages - hair
-
TYPE OF TEST :
-
LD50 - Lethal dose, 50 percent kill
-
ROUTE OF EXPOSURE :
-
Intravenous
-
SPECIES OBSERVED :
-
Rodent - mouse
-
DOSE/DURATION :
-
23200 ug/kg
-
TOXIC EFFECTS :
-
Behavioral - somnolence (general depressed activity) Gastrointestinal - hypermotility, diarrhea Skin and Appendages - hair
-
TYPE OF TEST :
-
LD50 - Lethal dose, 50 percent kill
-
ROUTE OF EXPOSURE :
-
Intraperitoneal
-
SPECIES OBSERVED :
-
Rodent - hamster
-
DOSE/DURATION :
-
1 mg/kg
-
TOXIC EFFECTS :
-
Details of toxic effects not reported other than lethal dose value
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Oral
-
SPECIES OBSERVED :
-
Rodent - rat
-
DOSE/DURATION :
-
3360 mg/kg/4W-C
-
TOXIC EFFECTS :
-
Liver - changes in liver weight Endocrine - changes in adrenal weight Biochemical - Enzyme inhibition, induction, or change in blood or tissue levels - transaminases
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Oral
-
DOSE :
-
200 ug/kg
-
SEX/DURATION :
-
lactating female 3 day(s) post-birth
-
TOXIC EFFECTS :
-
Reproductive - Maternal Effects - postpartum
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Oral
-
DOSE :
-
1 mg/kg
-
SEX/DURATION :
-
male 1 day(s) pre-mating
-
TOXIC EFFECTS :
-
Reproductive - Paternal Effects - spermatogenesis (incl. genetic material, sperm morphology, motility, and count)
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Intravenous
-
DOSE :
-
16800 ug/kg
-
SEX/DURATION :
-
female 10 week(s) after conception
-
TOXIC EFFECTS :
-
Reproductive - Fertility - abortion
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Intravenous
-
DOSE :
-
100 ng/kg
-
SEX/DURATION :
-
female 23 week(s) after conception
-
TOXIC EFFECTS :
-
Reproductive - Fertility - abortion
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Intravenous
-
DOSE :
-
65 ug/kg
-
SEX/DURATION :
-
female 27 week(s) after conception
-
TOXIC EFFECTS :
-
Reproductive - Fertility - abortion
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Intravenous
-
DOSE :
-
14400 ng/kg
-
SEX/DURATION :
-
female 15 week(s) after conception
-
TOXIC EFFECTS :
-
Reproductive - Fertility - abortion
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Intravenous
-
DOSE :
-
500 ng/kg
-
SEX/DURATION :
-
female 8 week(s) after conception
-
TOXIC EFFECTS :
-
Reproductive - Fertility - abortion
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Intravenous
-
DOSE :
-
400 ug/kg
-
SEX/DURATION :
-
female 16 week(s) after conception
-
TOXIC EFFECTS :
-
Reproductive - Maternal Effects - uterus, cervix, vagina
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Intraplacental
-
DOSE :
-
100 ug/kg
-
SEX/DURATION :
-
female 15 week(s) after conception
-
TOXIC EFFECTS :
-
Reproductive - Fertility - abortion
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Intracervical
-
DOSE :
-
10 ug/kg
-
SEX/DURATION :
-
female 42 week(s) after conception
-
TOXIC EFFECTS :
-
Reproductive - Maternal Effects - uterus, cervix, vagina
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Intracervical
-
DOSE :
-
48 ug/kg
-
SEX/DURATION :
-
female 14 week(s) after conception
-
TOXIC EFFECTS :
-
Reproductive - Fertility - abortion
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Intravaginal
-
DOSE :
-
2400 ug/kg
-
SEX/DURATION :
-
female 14 week(s) after conception
-
TOXIC EFFECTS :
-
Reproductive - Fertility - abortion
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Intravaginal
-
DOSE :
-
800 ug/kg
-
SEX/DURATION :
-
female 2 week(s) after conception
-
TOXIC EFFECTS :
-
Reproductive - Fertility - other measures of fertility
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Oral
-
DOSE :
-
1440 mg/kg
-
SEX/DURATION :
-
female 9-14 day(s) after conception
-
TOXIC EFFECTS :
-
Reproductive - Fertility - post-implantation mortality (e.g. dead and/or resorbed implants per total number of implants)
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Intravaginal
-
DOSE :
-
60 ug/kg
-
SEX/DURATION :
-
female 41 week(s) after conception
-
TOXIC EFFECTS :
-
Reproductive - Effects on Newborn - stillbirth
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Intraperitoneal
-
DOSE :
-
3600 ug/kg
-
SEX/DURATION :
-
female 20 day(s) after conception
-
TOXIC EFFECTS :
-
Reproductive - Maternal Effects - parturition
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Intraperitoneal
-
DOSE :
-
6 mg/kg
-
SEX/DURATION :
-
female 12-15 day(s) after conception
-
TOXIC EFFECTS :
-
Reproductive - Specific Developmental Abnormalities - homeostasis
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Intraperitoneal
-
DOSE :
-
75 mg/kg
-
SEX/DURATION :
-
female 6-10 day(s) after conception
-
TOXIC EFFECTS :
-
Reproductive - Specific Developmental Abnormalities - Central Nervous System Reproductive - Specific Developmental Abnormalities - eye/ear Reproductive - Specific Developmental Abnormalities - craniofacial (including nose and tongue)
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Intraperitoneal
-
DOSE :
-
1200 ug/kg
-
SEX/DURATION :
-
female 9-14 day(s) after conception
-
TOXIC EFFECTS :
-
Reproductive - Effects on Embryo or Fetus - fetotoxicity (except death, e.g., stunted fetus)
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Intraperitoneal
-
DOSE :
-
2 mg/kg
-
SEX/DURATION :
-
female 15 day(s) after conception
-
TOXIC EFFECTS :
-
Reproductive - Fertility - abortion
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Intraperitoneal
-
DOSE :
-
6 mg/kg
-
SEX/DURATION :
-
female 12-15 day(s) after conception
-
TOXIC EFFECTS :
-
Reproductive - Specific Developmental Abnormalities - homeostasis Reproductive - Specific Developmental Abnormalities - other developmental abnormalities
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Subcutaneous
-
DOSE :
-
17500 ug/kg
-
SEX/DURATION :
-
female 1-7 day(s) after conception
-
TOXIC EFFECTS :
-
Reproductive - Fertility - pre-implantation mortality (e.g. reduction in number of implants per female; total number of implants per corpora lutea)
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Subcutaneous
-
DOSE :
-
30 mg/kg
-
SEX/DURATION :
-
female 1-6 day(s) after conception
-
TOXIC EFFECTS :
-
Reproductive - Fertility - female fertility index (e.g. # females pregnant per # sperm positive females; # females pregnant per # females mated)
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Subcutaneous
-
DOSE :
-
32 mg/kg
-
SEX/DURATION :
-
female 4-7 day(s) after conception
-
TOXIC EFFECTS :
-
Reproductive - Fertility - post-implantation mortality (e.g. dead and/or resorbed implants per total number of implants)
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Subcutaneous
-
DOSE :
-
30 mg/kg
-
SEX/DURATION :
-
female 1-5 day(s) after conception
-
TOXIC EFFECTS :
-
Reproductive - Fertility - other measures of fertility
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Subcutaneous
-
DOSE :
-
64 mg/kg
-
SEX/DURATION :
-
female 4-7 day(s) after conception
-
TOXIC EFFECTS :
-
Reproductive - Fertility - female fertility index (e.g. # females pregnant per # sperm positive females; # females pregnant per # females mated)
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Intratesticular
-
DOSE :
-
12 mg/kg
-
SEX/DURATION :
-
male 3 day(s) pre-mating
-
TOXIC EFFECTS :
-
Reproductive - Paternal Effects - prostate, seminal vesicle, Cowper's gland, accessory glands
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Intratesticular
-
DOSE :
-
4 mg/kg
-
SEX/DURATION :
-
male 1 day(s) pre-mating
-
TOXIC EFFECTS :
-
Reproductive - Paternal Effects - testes, epididymis, sperm duct
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Intratesticular
-
DOSE :
-
400 ug/kg
-
SEX/DURATION :
-
male 1 day(s) pre-mating
-
TOXIC EFFECTS :
-
Reproductive - Paternal Effects - spermatogenesis (incl. genetic material, sperm morphology, motility, and count)
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Intrauterine
-
DOSE :
-
25 ug/kg
-
SEX/DURATION :
-
female 5 day(s) after conception
-
TOXIC EFFECTS :
-
Reproductive - Fertility - pre-implantation mortality (e.g. reduction in number of implants per female; total number of implants per corpora lutea)
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Oral
-
DOSE :
-
36 mg/kg
-
SEX/DURATION :
-
female 7-12 day(s) after conception
-
TOXIC EFFECTS :
-
Reproductive - Fertility - post-implantation mortality (e.g. dead and/or resorbed implants per total number of implants) Reproductive - Effects on Embryo or Fetus - fetal death
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Oral
-
DOSE :
-
1440 mg/kg
-
SEX/DURATION :
-
female 7-12 day(s) after conception
-
TOXIC EFFECTS :
-
Reproductive - Effects on Newborn - sex ratio
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Oral
-
DOSE :
-
720 mg/kg
-
SEX/DURATION :
-
female 7-12 day(s) after conception
-
TOXIC EFFECTS :
-
Reproductive - Specific Developmental Abnormalities - musculoskeletal system
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Intraperitoneal
-
DOSE :
-
30 mg/kg
-
SEX/DURATION :
-
female 9-14 day(s) after conception
-
TOXIC EFFECTS :
-
Reproductive - Fertility - post-implantation mortality (e.g. dead and/or resorbed implants per total number of implants) Reproductive - Fertility - litter size (e.g. # fetuses per litter; measured before birth)
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Intraperitoneal
-
DOSE :
-
60 mg/kg
-
SEX/DURATION :
-
female 9-14 day(s) after conception
-
TOXIC EFFECTS :
-
Reproductive - Fertility - abortion
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Intraperitoneal
-
DOSE :
-
150 mg/kg
-
SEX/DURATION :
-
female 6-10 day(s) after conception
-
TOXIC EFFECTS :
-
Reproductive - Specific Developmental Abnormalities - musculoskeletal system
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Subcutaneous
-
DOSE :
-
5 mg/kg
-
SEX/DURATION :
-
female 16 day(s) after conception
-
TOXIC EFFECTS :
-
Reproductive - Effects on Embryo or Fetus - fetal death
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Subcutaneous
-
DOSE :
-
746 ug/kg
-
SEX/DURATION :
-
female 12 day(s) after conception
-
TOXIC EFFECTS :
-
Reproductive - Specific Developmental Abnormalities - Central Nervous System Reproductive - Specific Developmental Abnormalities - eye/ear Reproductive - Specific Developmental Abnormalities - urogenital system
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Subcutaneous
-
DOSE :
-
746 ug/kg
-
SEX/DURATION :
-
female 12 day(s) after conception
-
TOXIC EFFECTS :
-
Reproductive - Effects on Embryo or Fetus - fetotoxicity (except death, e.g., stunted fetus)
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Subcutaneous
-
DOSE :
-
30 mg/kg
-
SEX/DURATION :
-
male 15 day(s) pre-mating
-
TOXIC EFFECTS :
-
Reproductive - Paternal Effects - spermatogenesis (incl. genetic material, sperm morphology, motility, and count)
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Subcutaneous
-
DOSE :
-
2 mg/kg
-
SEX/DURATION :
-
female 5-6 day(s) after conception
-
TOXIC EFFECTS :
-
Reproductive - Fertility - other measures of fertility
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Subcutaneous
-
DOSE :
-
20 ug/kg
-
SEX/DURATION :
-
female 16 day(s) after conception
-
TOXIC EFFECTS :
-
Reproductive - Fertility - abortion
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Subcutaneous
-
DOSE :
-
18 mg/kg
-
SEX/DURATION :
-
female 21-22 day(s) after conception
-
TOXIC EFFECTS :
-
Reproductive - Fertility - abortion
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Subcutaneous
-
DOSE :
-
3 mg/kg
-
SEX/DURATION :
-
female 1 day(s) after conception
-
TOXIC EFFECTS :
-
Reproductive - Fertility - other measures of fertility
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Subcutaneous
-
DOSE :
-
22500 ug/kg
-
SEX/DURATION :
-
female 1-3 day(s) after conception
-
TOXIC EFFECTS :
-
Reproductive - Fertility - female fertility index (e.g. # females pregnant per # sperm positive females; # females pregnant per # females mated) Reproductive - Fertility - pre-implantation mortality (e.g. reduction in number of implants per female; total number of implants per corpora lutea)
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Intravenous
-
DOSE :
-
2 mg/kg
-
SEX/DURATION :
-
female 1 day(s) after conception
-
TOXIC EFFECTS :
-
Reproductive - Fertility - pre-implantation mortality (e.g. reduction in number of implants per female; total number of implants per corpora lutea) Reproductive - Fertility - litter size (e.g. # fetuses per litter; measured before birth)
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Subcutaneous
-
DOSE :
-
4 mg/kg
-
SEX/DURATION :
-
female 23-24 day(s) after conception
-
TOXIC EFFECTS :
-
Reproductive - Fertility - abortion
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Oral
-
DOSE :
-
48 mg/kg
-
SEX/DURATION :
-
female 4-6 day(s) after conception
-
TOXIC EFFECTS :
-
Reproductive - Fertility - female fertility index (e.g. # females pregnant per # sperm positive females; # females pregnant per # females mated)
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Intraperitoneal
-
DOSE :
-
200 ug/kg
-
SEX/DURATION :
-
female 8 day(s) after conception
-
TOXIC EFFECTS :
-
Reproductive - Specific Developmental Abnormalities - musculoskeletal system
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Intraperitoneal
-
DOSE :
-
500 ug/kg
-
SEX/DURATION :
-
female 8 day(s) after conception
-
TOXIC EFFECTS :
-
Reproductive - Effects on Embryo or Fetus - fetotoxicity (except death, e.g., stunted fetus)
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Intraperitoneal
-
DOSE :
-
1 mg/kg
-
SEX/DURATION :
-
female 8 day(s) after conception
-
TOXIC EFFECTS :
-
Reproductive - Effects on Embryo or Fetus - fetal death
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Subcutaneous
-
DOSE :
-
800 ug/kg
-
SEX/DURATION :
-
female 8 day(s) after conception
-
TOXIC EFFECTS :
-
Reproductive - Effects on Embryo or Fetus - fetotoxicity (except death, e.g., stunted fetus) Reproductive - Specific Developmental Abnormalities - Central Nervous System Reproductive - Specific Developmental Abnormalities - musculoskeletal system
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Subcutaneous
-
DOSE :
-
800 ug/kg
-
SEX/DURATION :
-
female 8 day(s) after conception
-
TOXIC EFFECTS :
-
Reproductive - Specific Developmental Abnormalities - cardiovascular (circulatory) system Reproductive - Specific Developmental Abnormalities - urogenital system Reproductive - Specific Developmental Abnormalities - homeostasis
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Subcutaneous
-
DOSE :
-
800 ug/kg
-
SEX/DURATION :
-
female 8 day(s) after conception
-
TOXIC EFFECTS :
-
Reproductive - Fertility - post-implantation mortality (e.g. dead and/or resorbed implants per total number of implants)
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Subcutaneous
-
DOSE :
-
1600 ug/kg
-
SEX/DURATION :
-
female 5 day(s) after conception
-
TOXIC EFFECTS :
-
Reproductive - Fertility - other measures of fertility
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Subcutaneous
-
DOSE :
-
3600 ug/kg
-
SEX/DURATION :
-
female 4-6 day(s) after conception
-
TOXIC EFFECTS :
-
Reproductive - Maternal Effects - ovaries, fallopian tubes Reproductive - Fertility - female fertility index (e.g. # females pregnant per # sperm positive females; # females pregnant per # females mated)
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Intracervical
-
DOSE :
-
48 ug/kg
-
SEX/DURATION :
-
female 33-44 day(s) after conception
-
TOXIC EFFECTS :
-
Reproductive - Maternal Effects - uterus, cervix, vagina
-
TYPE OF TEST :
-
TDLo - Lowest published toxic dose
-
ROUTE OF EXPOSURE :
-
Intravaginal
-
DOSE :
-
4 ug/kg
-
SEX/DURATION :
-
female 1 day(s) pre-mating
-
TOXIC EFFECTS :
-
Reproductive - Maternal Effects - uterus, cervix, vagina
-
TYPE OF TEST :
-
Mutation test systems - not otherwise specified
-
TYPE OF TEST :
-
Sperm Morphology
MUTATION DATA
-
TYPE OF TEST :
-
Unscheduled DNA synthesis
-
TEST SYSTEM :
-
Rodent - guinea pig Lung
-
DOSE/DURATION :
-
1 mg/L
-
REFERENCE :
-
PSEBAA Proceedings of the Society for Experimental Biology and Medicine. (Academic Press, Inc., 1 E. First St., Duluth, MN 55802) V.1- 1903/04- Volume(issue)/page/year: 171,109,1982 *** REVIEWS *** TOXICOLOGY REVIEW CSPEAX Cosmetics and Perfumery. (Oak Park, IL) V.88-90, 1973-75. For publisher information, see CTOIDG. Volume(issue)/page/year: 90(12),28,1975 TOXICOLOGY REVIEW AJOGAH American Journal of Obstetrics and Gynecology. (C.V. Mosby Co., 11830 Westline Industrial Dr., St. Louis, MO 63146) V.1- 1920- Volume(issue)/page/year: 113,130,1972
|


![({5-[(3-METHOXYPHENYL)AMINO]-1,3,4-THIADIAZOL-2-YL}THIO)ACETICACID structure](https://image.chemsrc.com/caspic/175/104010-72-2.png)