(-)-Epicatechin gallate
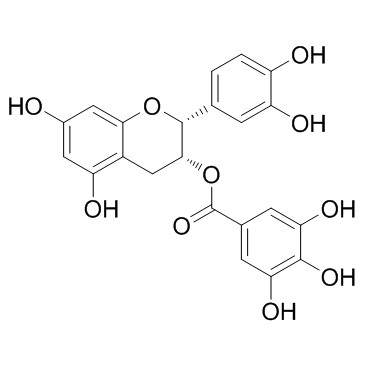
(-)-Epicatechin gallate structure
|
Common Name | (-)-Epicatechin gallate | ||
|---|---|---|---|---|
| CAS Number | 1257-08-5 | Molecular Weight | 442.372 | |
| Density | 1.8±0.1 g/cm3 | Boiling Point | 861.7±65.0 °C at 760 mmHg | |
| Molecular Formula | C22H18O10 | Melting Point | 257-258ºC | |
| MSDS | Chinese USA | Flash Point | 305.0±27.8 °C | |
Use of (-)-Epicatechin gallateEpicatechin gallate inhibits cyclooxygenase-1 (COX-1) with an IC50 of 7.5 μM. |
| Name | (-)-epicatechin-3-O-gallate |
|---|---|
| Synonym | More Synonyms |
| Description | Epicatechin gallate inhibits cyclooxygenase-1 (COX-1) with an IC50 of 7.5 μM. |
|---|---|
| Related Catalog | |
| Target |
COX-1:7.5 μM (IC50) |
| In Vitro | Epicatechin gallate exhibits >95% inhibitory activity at 70 μg/mL against cyclooxygenase-1 (COX-1) with an IC50 of 7.5 μM[1]. |
| In Vivo | Epicatechin gallate ((-)-Epicatechin-3-O-gallate, ECG), a component of Rhei Rhizoma, is one of the active components of Onpi-to, a herbal medicine composed of five crude drugs (Rhei Rhizome, Glycyrrhizae Radix, Ginseng Radix, Zingiberis Rhizoma and Aconiti Tuber). Following intravenous injection of Epicatechin gallate (1.0 mg/kg) in rats, the plasma concentration vs. time curve is fitted in a three compartment model. Pharmacokinetic parameters for plasma Epicatechin gallate (ECG) are measured. ECG has a t1/2α of 0.038 h, a t1/2βof 0.291 h and a t1/2γ of 4.033 h . The CLtot of ECG is 4.19 L/h • kg. The Vdss is 12.39 L/kg[2]. |
| Animal Admin | Rats[2] Male Sprague-Dawley rats, obtained at 7 weeks of age (210-245 g) are used. Epicatechin gallate (ECG) is suspended in 0.5% w/v sodium carboxymethylcellulose at 12.5, 25.0 and 50.0 mg/10 mL for oral administration to rats at 10 mL/kg. For intravenous injection in rats at 1.0 mg/kg, an ethanolic solution of Epicatechin gallate is diluted with 10% w/v sodium citrate solution to 1.0 mg/mL; the final concentration of ethanol is 1% v/v[2]. |
| References |
| Density | 1.8±0.1 g/cm3 |
|---|---|
| Boiling Point | 861.7±65.0 °C at 760 mmHg |
| Melting Point | 257-258ºC |
| Molecular Formula | C22H18O10 |
| Molecular Weight | 442.372 |
| Flash Point | 305.0±27.8 °C |
| Exact Mass | 442.089996 |
| PSA | 177.14000 |
| LogP | 2.67 |
| Vapour Pressure | 0.0±0.3 mmHg at 25°C |
| Index of Refraction | 1.825 |
| InChIKey | LSHVYAFMTMFKBA-TZIWHRDSSA-N |
| SMILES | O=C(OC1Cc2c(O)cc(O)cc2OC1c1ccc(O)c(O)c1)c1cc(O)c(O)c(O)c1 |
| Storage condition | 2-8°C |
CHEMICAL IDENTIFICATION
HEALTH HAZARD DATAACUTE TOXICITY DATA
|
| Personal Protective Equipment | Eyeshields;Gloves;type N95 (US);type P1 (EN143) respirator filter |
|---|---|
| Hazard Codes | Xn |
| RIDADR | NONH for all modes of transport |
| WGK Germany | 3 |
| RTECS | DH9030000 |
| Precursor 8 | |
|---|---|
| DownStream 10 | |
|
Influence of different withering conditions on phenolic composition of Avanà, Chatus and Nebbiolo grapes for the production of 'Reinforced' wines.
Food Chem. 194 , 247-56, (2015) The impact of postharvest withering rates on the phenolic composition of 'reinforced' wines produced with partially dehydrated grapes was evaluated. The study was performed on winegrape varieties with... |
|
|
Effects of UV exclusion on the physiology and phenolic composition of leaves and berries of Vitis vinifera cv. Graciano.
J. Sci. Food Agric. 95(2) , 409-16, (2014) Ultraviolet (UV) radiation induces adaptive responses that can be used for plant production improvement. The aim of this study was to assess the effect of solar UV exclusion on the physiology and phen... |
|
|
Identification of Vitis vinifera L. grape berry skin color mutants and polyphenolic profile.
Food Chem. 194 , 117-27, (2015) A germplasm set of twenty-five grapevine accessions, forming eleven groups of possible berry skin color mutants, were genotyped with twelve microsatellite loci, being eleven of them identified as true... |
|
Name: Antibacterial activity against Staphylococcus epidermidis ATCC 0155 after 24 hrs by N...
Source: ChEMBL
Target: Staphylococcus epidermidis
External Id: CHEMBL1115800
|
|
Name: Inhibition against HIV- reverse transcriptase in cell culture at 20 ug/mL
Source: ChEMBL
Target: Reverse transcriptase/RNaseH
External Id: CHEMBL689636
|
|
Name: Antibacterial activity against Micrococcus luteus ATCC 10240 after 24 hrs by NCCLS me...
Source: ChEMBL
Target: Micrococcus luteus
External Id: CHEMBL1115799
|
|
Name: Inhibition of FabZ
Source: ChEMBL
Target: 3-hydroxyacyl-[acyl-carrier-protein] dehydratase
External Id: CHEMBL859169
|
|
Name: Antibacterial activity against Staphylococcus aureus ATCC 25923 after 24 hrs by NCCLS...
Source: ChEMBL
Target: Staphylococcus aureus
External Id: CHEMBL1115798
|
|
Name: Inhibition of FabI
Source: ChEMBL
Target: Enoyl-acyl-carrier protein reductase
External Id: CHEMBL859170
|
|
Name: Antibacterial activity against Enterococcus faecalis ATCC 29212 after 24 hrs by NCCLS...
Source: ChEMBL
Target: Enterococcus faecalis
External Id: CHEMBL1115797
|
|
Name: Binding affinity to BCL2
Source: ChEMBL
Target: Apoptosis regulator Bcl-2
External Id: CHEMBL937576
|
|
Name: Antifungal activity against Candida albicans ATCC 10231 after 24 to 72 hrs by NCCLS m...
Source: ChEMBL
Target: Candida albicans
External Id: CHEMBL1115808
|
|
Name: Dual reporter qHTS for antagonist of Pregnane X Receptor (hPXR) against the NCATS DSH...
Source: NCGC
External Id: DSHEA-v1-PXR-antagonist-CTF
|
| Benzoic acid, 3,4,5- trihydroxy-, 2-(3,4-dihydroxyphenyl)-3,4-dihydro-5,7-dihydroxy-2H-1-benzopyran-3-ylester, (2R-cis)-, |
| MFCD00075936 |
| Epicatechol, 3-gallate, (-)- (8CI) |
| ECG |
| (-)-cis-3,3',4',5,7-Pentahydroxyflavane 3-gallate |
| Benzoic acid, 3,4,5-trihydroxy-, (2R,3R)-2-(3,4-dihydroxyphenyl)-3,4-dihydro-5,7-dihydroxy-2H-1-benzopyran-3-yl ester |
| (−)-Epicatechin gallate,from green tea |
| (2R,3R)-2-(3,4-Dihydroxyphenyl)-5,7-dihydroxy-3,4-dihydro-2H-chromen-3-yl 3,4,5-trihydroxybenzoate |
| Epicatechin gallate |
| (-)-epicatechin-3-gallate |
| (-)-Epicatechingallate |
| (-)-epicatechin gallate |
| (−)-Epicatechin gallate |
| (-)-cis-2-(3,4-Dihydroxyphenyl)-3,4-dihydro-1(2H)-benzopyran-3,5,7-triol 3-gallate |
 CAS#:63604-98-8
CAS#:63604-98-8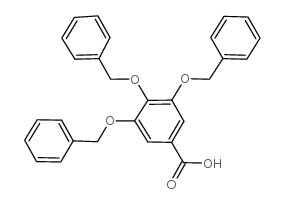 CAS#:1486-48-2
CAS#:1486-48-2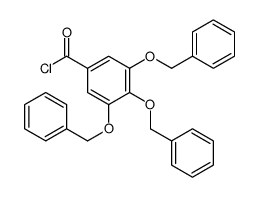 CAS#:1486-47-1
CAS#:1486-47-1![(2R-trans)-2-[3,4-bis(phenylmethoxy)phenyl]-3,4-dihydro-5,7-bis(phenylmethoxy)-2H-1-benzopyran-3-ol Structure](https://image.chemsrc.com/caspic/113/20728-73-8.png) CAS#:20728-73-8
CAS#:20728-73-8 CAS#:87292-49-7
CAS#:87292-49-7![(2R)-2-[3,4-bis(phenylmethoxy)phenyl]-5,7-bis(phenylmethoxy)-4H-chromen-3-one Structure](https://image.chemsrc.com/caspic/087/87292-54-4.png) CAS#:87292-54-4
CAS#:87292-54-4![(E)-3-[2,4-bis(benzyloxy)-6-hydroxyphenyl]-1-[3,4-bis(benzyloxy)phenyl]propene Structure](https://image.chemsrc.com/caspic/196/732298-08-7.png) CAS#:732298-08-7
CAS#:732298-08-7![(+)-(1S,2S)-3-[2,4-bis(benzyloxy)-6-hydroxyphenyl]-1-[3,4-bis(benzyloxy)phenyl]propane-1,2-diol Structure](https://image.chemsrc.com/caspic/255/732298-11-2.png) CAS#:732298-11-2
CAS#:732298-11-2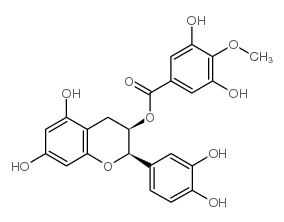 CAS#:108907-44-4
CAS#:108907-44-4 CAS#:1707-75-1
CAS#:1707-75-1 CAS#:621-54-5
CAS#:621-54-5 CAS#:31129-95-0
CAS#:31129-95-0 CAS#:31129-94-9
CAS#:31129-94-9![2(3H)-Furanone,5-[(3,4-dihydroxyphenyl)methyl]dihydro-,(5R)-(9CI) structure](https://image.chemsrc.com/caspic/408/191666-22-5.png) CAS#:191666-22-5
CAS#:191666-22-5 CAS#:149-91-7
CAS#:149-91-7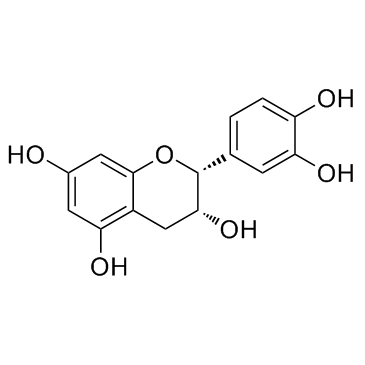 CAS#:490-46-0
CAS#:490-46-0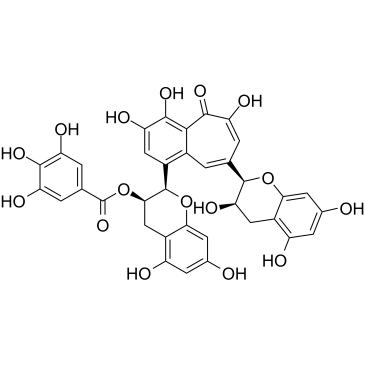 CAS#:28543-07-9
CAS#:28543-07-9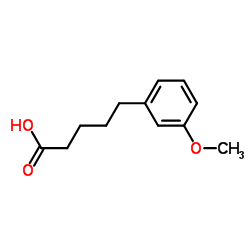 CAS#:6500-64-7
CAS#:6500-64-7
